正在加载图片...
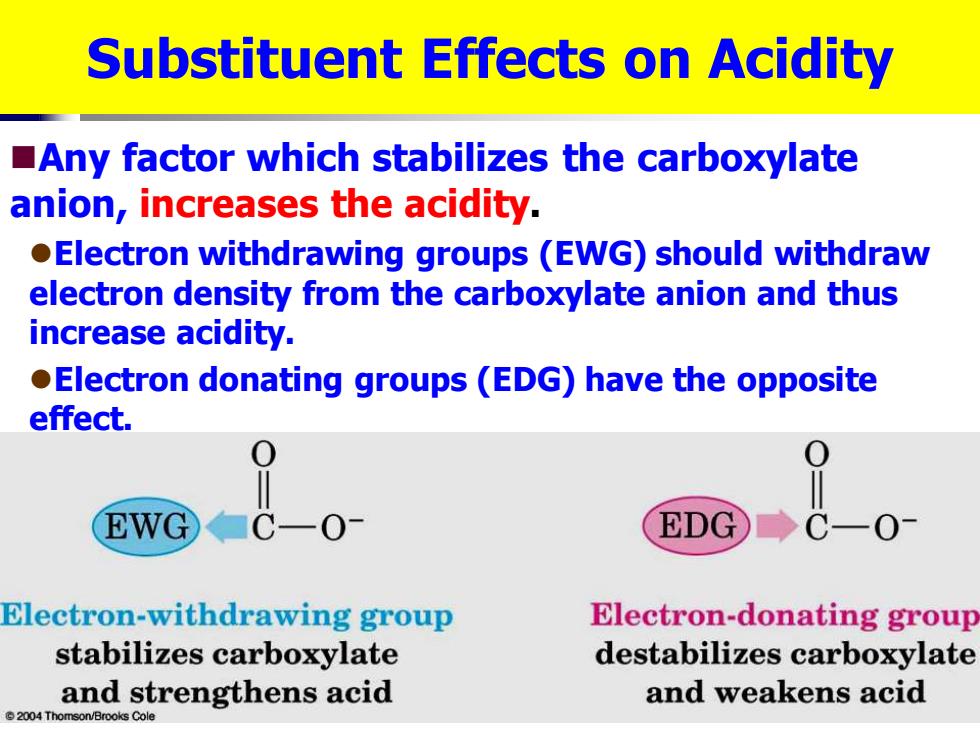
Substituent Effects on Acidity Any factor which stabilizes the carboxylate anion,increases the acidity. Electron withdrawing groups (EWG)should withdraw electron density from the carboxylate anion and thus increase acidity. Electron donating groups(EDG)have the opposite effect. EWG ■CO1 EDG C-0 Electron-withdrawing group Electron-donating group stabilizes carboxylate destabilizes carboxylate and strengthens acid and weakens acid 2004 Thomson/Brooks Cole Substituent Effects on Acidity ◼Any factor which stabilizes the carboxylate anion, increases the acidity. ⚫Electron withdrawing groups (EWG) should withdraw electron density from the carboxylate anion and thus increase acidity. ⚫Electron donating groups (EDG) have the opposite effect