正在加载图片...
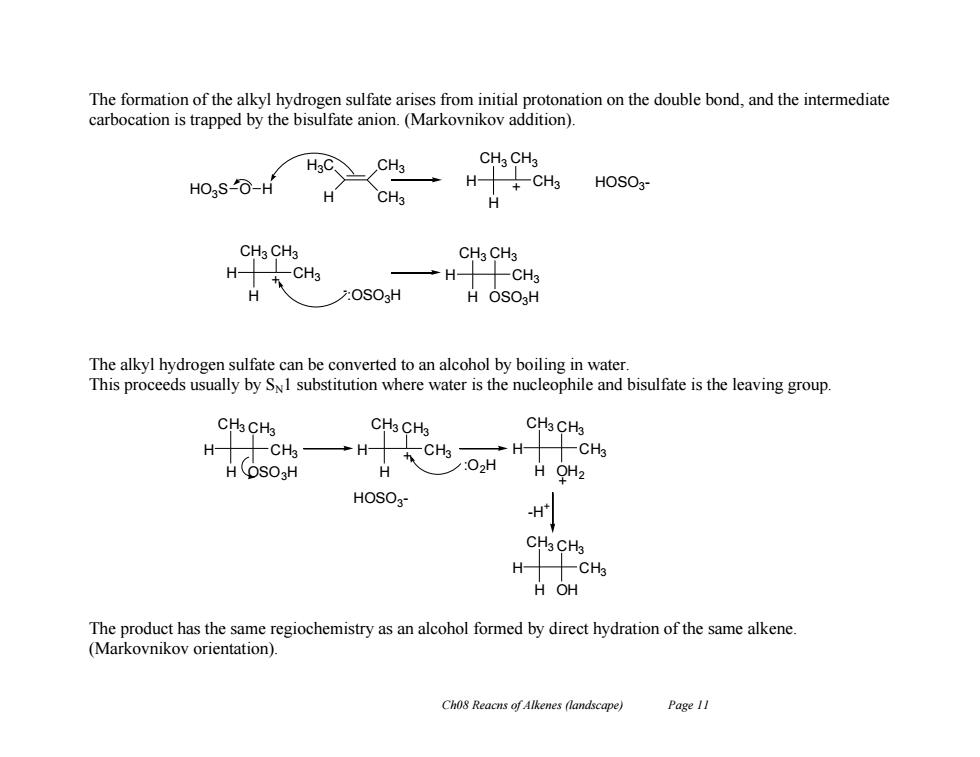
The formation of the alkyl hydrogen sulfate arises from initial protonation on the double bond,and the intermediate carbocation is trapped by the bisulfate anion.(Markovnikov addition). CH3 CHa CH3 HO3SO-H →H+CHs HOSO3- H CH3 CH3 CH3 CH3 CH3 H CH H CH H :OSO3H H OSO H The alkyl hydrogen sulfate can be converted to an alcohol by boiling in water. This proceeds usually by SI substitution where water is the nucleophile and bisulfate is the leaving group. CH3CH3 CH3 CH3 CH3CH3 →H 车CH →HCHs HOSO3H H /:02H H OH2 HOSO3 H CH3CH3 H-CHs H OH The product has the same regiochemistry as an alcohol formed by direct hydration of the same alkene (Markovnikov orientation). Ch08 Reacns of Alkenes (landscape) Page 11Ch08 Reacns of Alkenes (landscape) Page 11 The formation of the alkyl hydrogen sulfate arises from initial protonation on the double bond, and the intermediate carbocation is trapped by the bisulfate anion. (Markovnikov addition). The alkyl hydrogen sulfate can be converted to an alcohol by boiling in water. This proceeds usually by SN1 substitution where water is the nucleophile and bisulfate is the leaving group. The product has the same regiochemistry as an alcohol formed by direct hydration of the same alkene. (Markovnikov orientation). H3C H CH3 CH3 H CH3 H CH3 CH3 H CH3 H CH3 CH3 H CH3 H CH3 CH3 OSO3H HO3S O H + HOSO3 - - :OSO3H + H CH3 H CH3 CH3 OSO3H H CH3 H CH3 CH3 HOSO3 - + :O2H H CH3 H CH3 CH3 OH2 + -H + H CH3 H CH3 CH3 OH