正在加载图片...
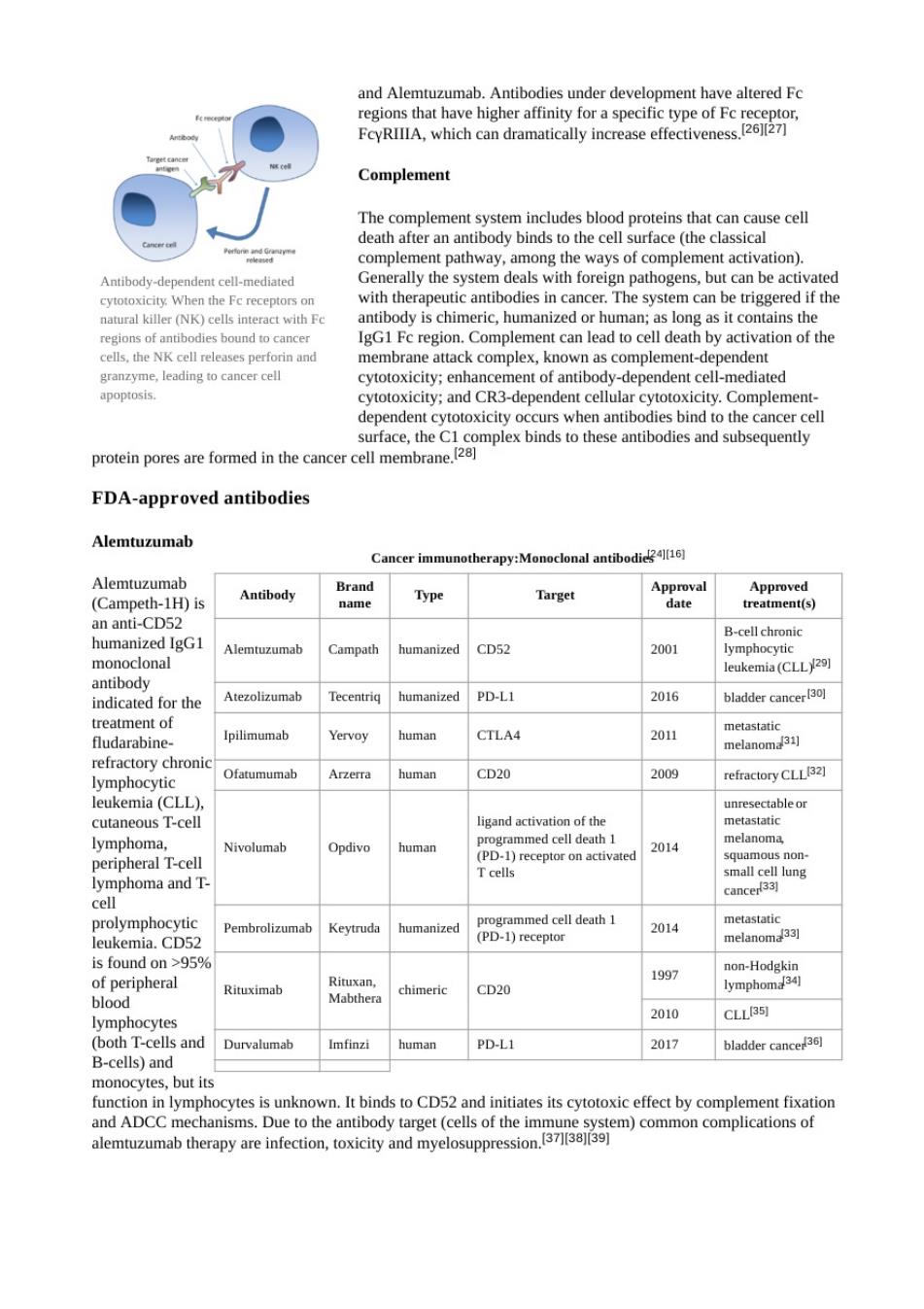
and alemtuzumab.antibodies under development have altered fc 将m代 Complement complement pathway,among the ways of complement activation). Antibody- ndent cell-mediated When the Ferecep Generally the systemr pathogens,but can be act the is chi ies in cance The system can be IgG1 Fc region.Com cells,the NK cel releses perforinand membrane attack complex,known as complement-dependent granzyme eadng tocancer cel r cytotox proeomplex binds to these antibodies and subseqny FDA-approved antibodies Alemtuzumab Cancer immunotherapy:Monoclonal antibodi Alemtuzu (Cam eth-1H)is Antibody Brand Type Approval m) an anti-CD52 humanized IgG1 CD52 2001 mo leukemia (CLL29) indicated for the Atezolizumab humanized PD-LI 2016 bladder cance treatment of fludarabine Ipilimumab Yervoy human CTLA4 2011 refractory chroni Ofatumumal human CD20 2009 refractory CLLB2] 1 eukemia(CLL】 cutaneous T-cell lymphoma. Nivolumab Opdivo human 2014 mall cell lur Pembrolizumab 2014 leukemia.CD5. 95 197 non-Hodgkin Rituximab Mabthera chimeric CD20 lymphom 2010 CLLB51 lymphocytes T-cells and Durvalumab Imfinzi human PD-L1 2017 bladder cancer3同 ells)an oyeiskIbinds to CD52dtcyotoxiefect by come ixio aeoaa