正在加载图片...
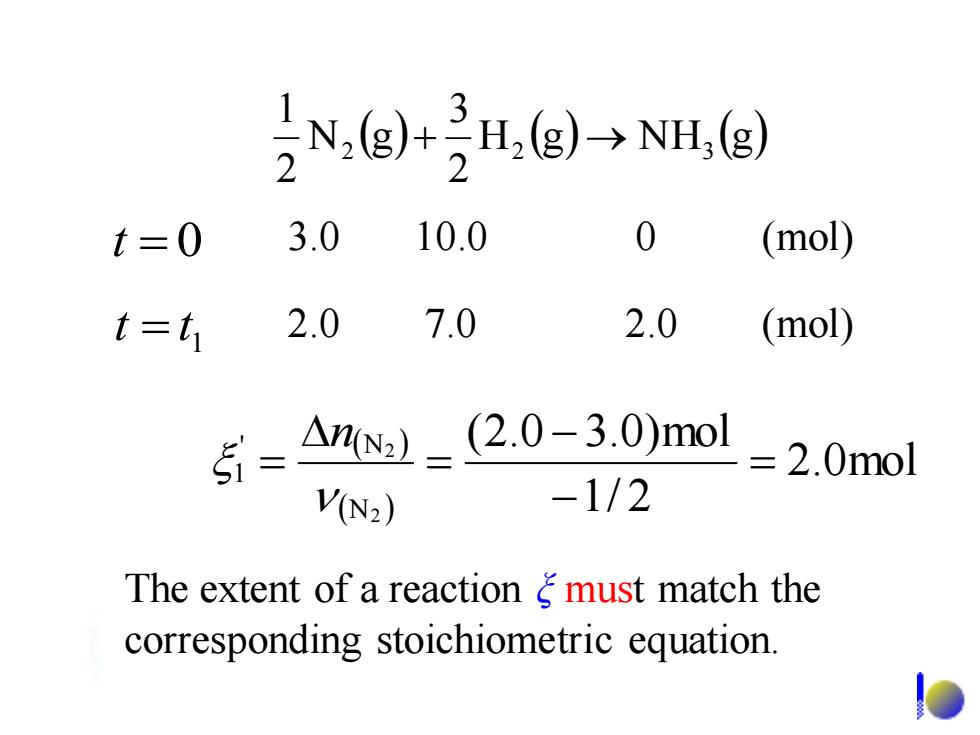
5N,(g+H,(g)→NI,g) t=0 3.0 10.0 0 (mol) f= t 2.0 7.0 2.0 (mol) 5-An:1=(2.0-3.0)mol =2.0mol V(N2) -1/2 The extent of a reaction g must match the corresponding stoichiometric equation. The extent of a reaction ξ must match the corresponding stoichiometric equation. ( ) H (g) N H (g) 2 3 N g 2 1 2 + 2 → 3 ( ) ( ) 2.0mol 1/ 2 (2.0 3.0)mol 2 2 N ' N 1 = − − = = n 1 t = t 2.0 7.0 2.0 (mol) t = 0 3.0 10.0 0 (mol)