正在加载图片...
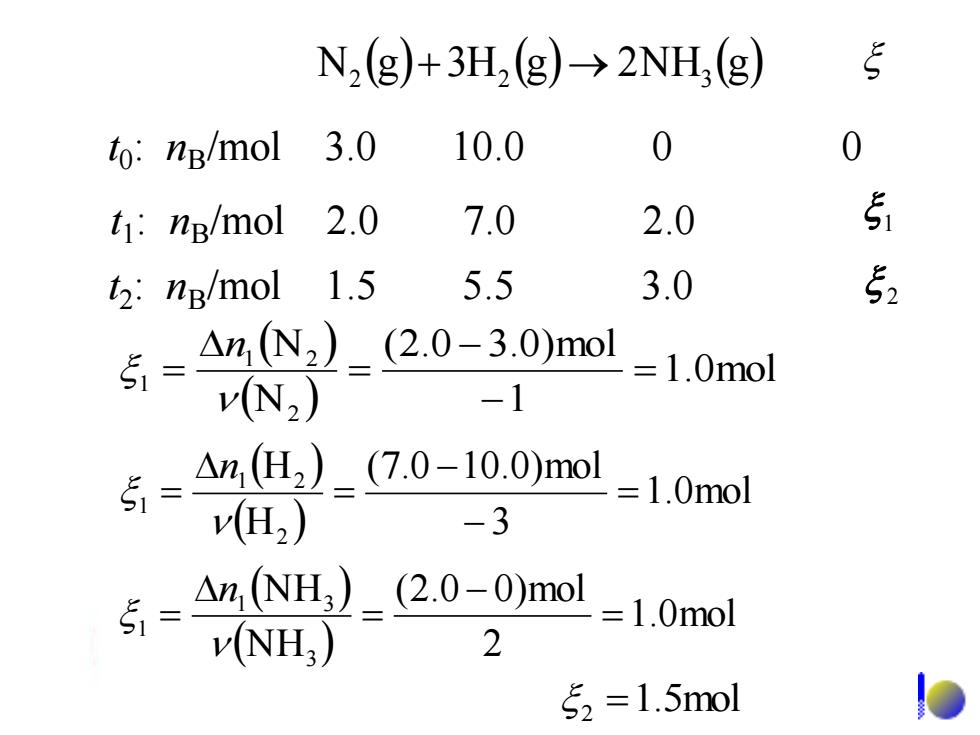
N2g)+3H2(g)-→2NH,(g) to:ng/mol 3.0 10.0 0 0 t1:ng/mol 2.0 7.0 2.0 51 t2:ng/mol 1.5 5.5 3.0 52 5=AmN2_2.0-3.0mol vN2) =1.0mol -1 $ △n2)_(7.0-10.0)mo1 v(H2) -3 =1.0mol △n,NH)_(2.0-0)mol v(NH) 2 =1.0mol 52=1.5mol N (g) 3H (g) 2NH (g) 2 + 2 → 3 t0 : nB/mol 3.0 10.0 0 0 t1 : nB/mol 2.0 7.0 2.0 t2 : nB/mol 1.5 5.5 3.0 1 2 ( ) ( ) 1.0mol 1 (2.0 3.0)mol N N 2 1 2 1 = − − = = n 1.5mol 2 = ( ) ( ) 1.0mol 2 (2.0 0)mol N H N H 3 1 3 1 = − = = n ( ) ( ) 1.0mol 3 (7.0 10.0)mol H H 2 1 2 1 = − − = = n