正在加载图片...
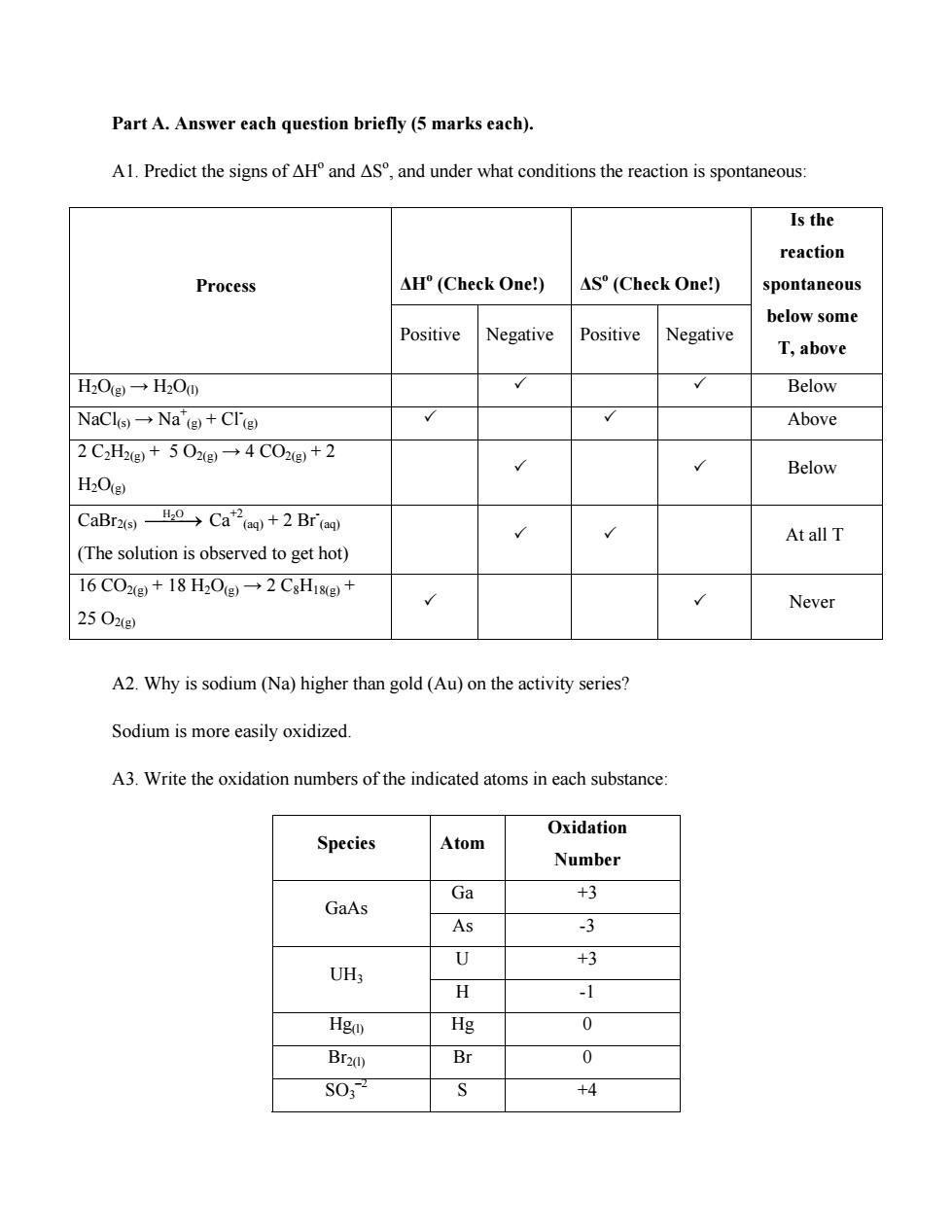
Part A.Answer each question briefly(5 marks each). Al Predict the signs of aH and as and under what conditions the reaction is spontaneous Is the reaction Process AH°(Check One) AS"(Check One!) spontaneous below some Positive Negative Positive Negative T,above HOg→HO0 Below NaCls→Nag+CTg Above 2C2H2g+502g→4C02g+2 Below H2O() AtallT (The solution is observed to get hot) 16 CO2()+18 H2O(g)-2 CsHi8g)+ Never 2502e A2.Why is sodium(Na)higher than gold (Au)on the activity series? Sodium is more easily oxidized. A3.Write the oxidation numbers of the indicated atoms in each substance Oxidation Species Atom Number Ga +3 GaAs As 3 U +3 UH3 -1 Hgo) 0 Br20) Br 0 SO; +4 Part A. Answer each question briefly (5 marks each). A1. Predict the signs of ΔHo and ΔSo , and under what conditions the reaction is spontaneous: ΔHo (Check One!) ΔSo Process (Check One!) Positive Negative Positive Negative Is the reaction spontaneous below some T, above H2O(g) → H2O(l) 3 3 Below NaCl(s) → Na+ (g) + Cl- (g) 3 3 Above 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g) 3 3 Below CaBr2(s) ⎯H O ⎯⎯2 → Ca+2 (aq) + 2 Br- (aq) (The solution is observed to get hot) 3 3 At all T 16 CO2(g) + 18 H2O(g) → 2 C8H18(g) + 25 O2(g) 3 3 Never A2. Why is sodium (Na) higher than gold (Au) on the activity series? Sodium is more easily oxidized. A3. Write the oxidation numbers of the indicated atoms in each substance: Species Atom Oxidation Number Ga +3 GaAs As -3 U +3 UH3 H -1 Hg(l) Hg 0 Br2(l) Br 0 SO3 –2 S +4