正在加载图片...
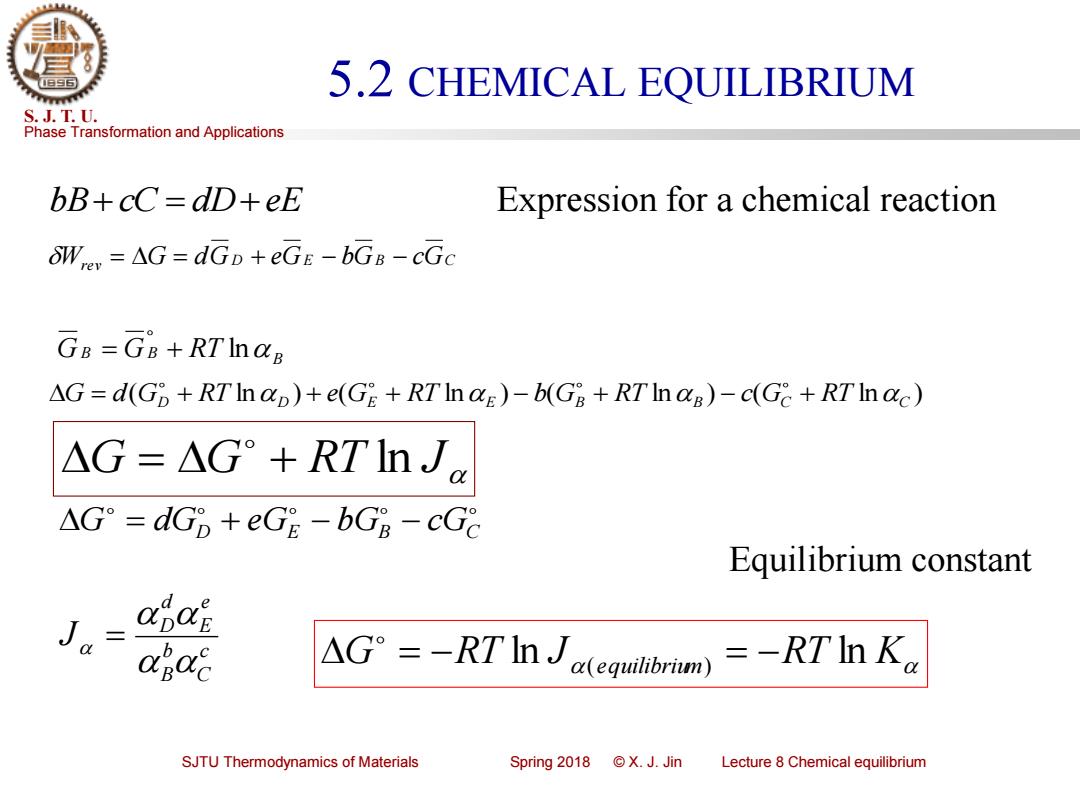
5.2 CHEMICAL EQUILIBRIUM S.J.T.U. Phase Transformation and Applications bB+cC=dD+eE Expression for a chemical reaction OWrer =AG=dGD+eGE-bGB-cGc Ge=GB+RTInag AG=d(Gp+RTInap)+e(GE +RTInaE)-b(G8+RTIn aB)-c(Gc+RTInac) △G=△G°+RTIn Je △G°=dG8+eGE-bGB-cG8 Equilibrium constant de Ja ODOE b △G°=-RTIJaerm)=-RTIn K2 SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibriumPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium 5.2 CHEMICAL EQUILIBRIUM bB+ cC = dD+ eE Wrev = G = dGD + eGE − bGB − cGC ( ln ) ( ln ) ( ln ) ( ln ) D D E E B B GC RT C G = d G + RT + e G + RT − b G + RT − c + G = G + RT ln J D E B C G = dG + eG − bG − cG c C b B e E d D J = B GB = GB + RT ln RT K G RT J equilibrium = − ln ( ) = − ln Equilibrium constant Expression for a chemical reaction