正在加载图片...
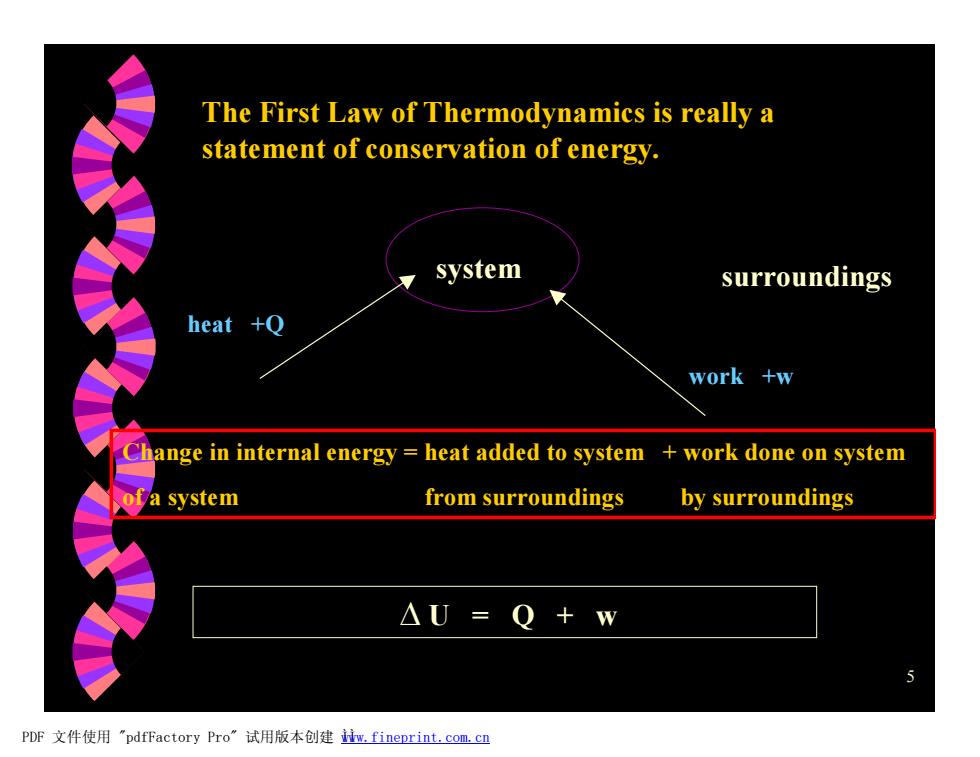
The First Law of Thermodynamics is really a statement of conservation of energy. system surroundings heat +Q work +w Change in internal energy heat added to system work done on system of a system from surroundings by surroundings △U=Q+w PDF文件使用"pdfFactory Pro”试用版本创建iw,fineprint.com.cn 5 The First Law of Thermodynamics is really a statement of conservation of energy. system surroundings heat +Q work +w Change in internal energy = heat added to system + work done on system of a system from surroundings by surroundings D U = Q + w PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn Ì