正在加载图片...
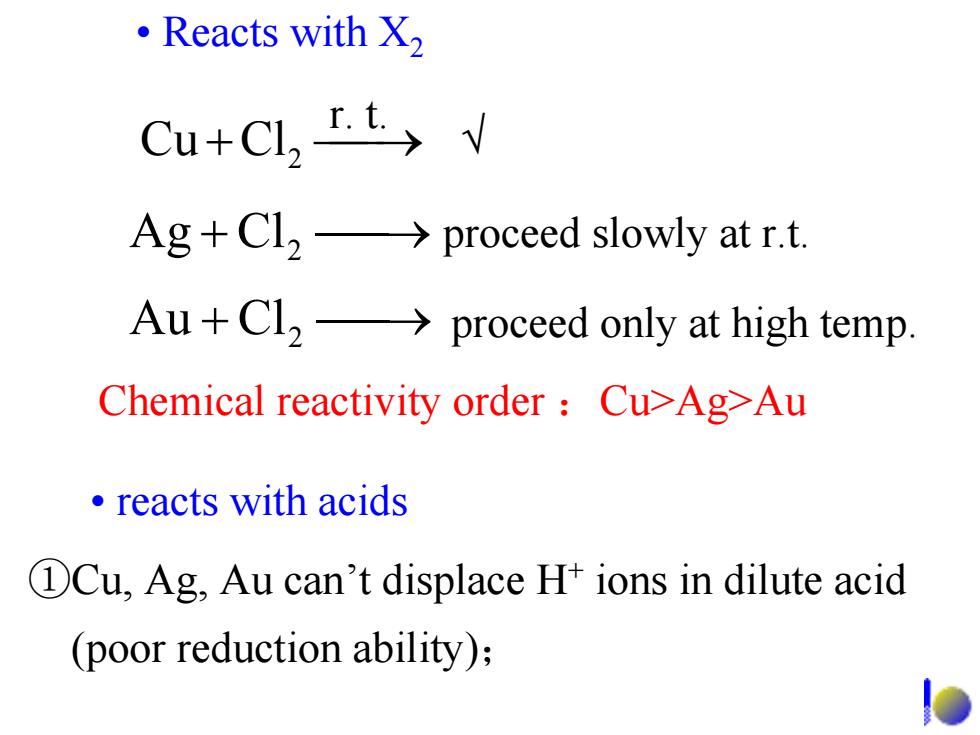
·Reacts with X) Cu+Cl,I.t> Ag+Cl2->proceed slowly at r.t. Au+Cl2->proceed only at high temp. Chemical reactivity order Cu>Ag-Au ·reacts with acids DCu,Ag,Au can't displace H+ions in dilute acid (poor reduction ability); • Reacts with X2 ClCu 2 ⎯+ ⎯→r. t. Chemical reactivity order :Cu>Ag>Au ⎯+ ⎯→ proceed only at high temp. ClAu 2 ⎯+ ⎯→ proceed slowly at r.t. ClAg 2 • reacts with acids ①Cu, Ag, Au can’t displace H+ ions in dilute acid (poor reduction ability); √