正在加载图片...
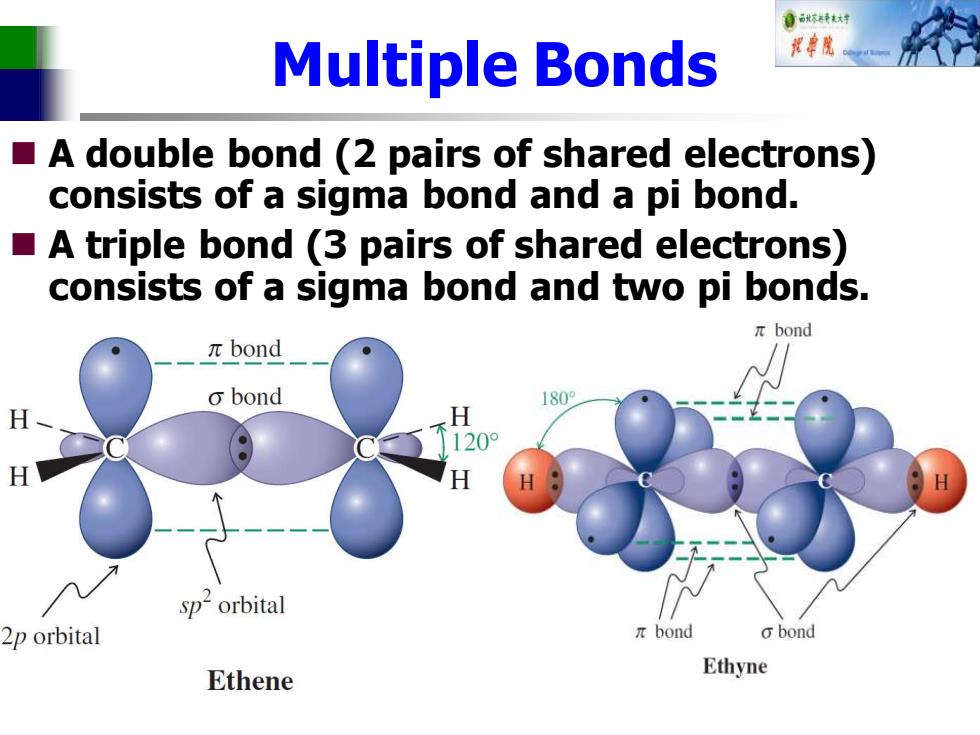
自标转对 Multiple Bonds A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds. πbond πbond o bond 1809 H H 8 120° H H sp2orbital 2p orbital πbond obond Ethene Ethyne Multiple Bonds ◼ A double bond (2 pairs of shared electrons) consists of a sigma bond and a pi bond. ◼ A triple bond (3 pairs of shared electrons) consists of a sigma bond and two pi bonds