正在加载图片...
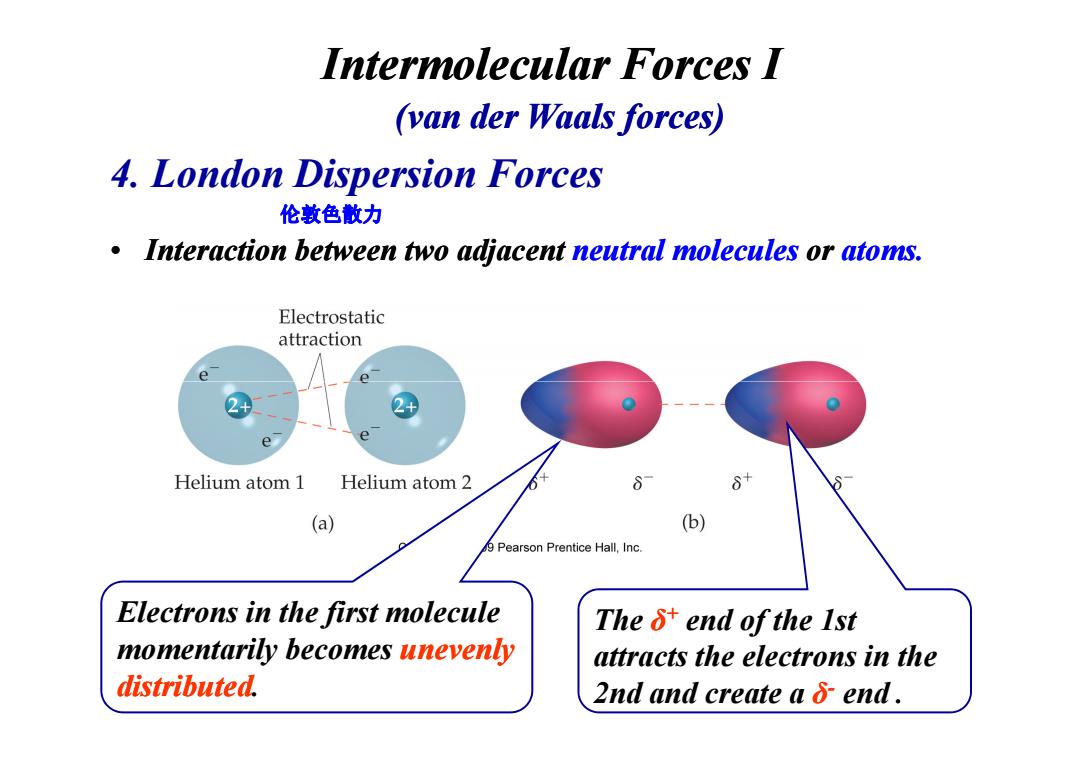
Intermolecular Forces I (van der Waals forces) 4.London Dispersion Forces 伦敦色散力 .Interaction between two adjacent neutral molecules or atoms. Electrostatic attraction Helium atom 1 Helium atom 2 (a) (b) 9 Pearson Prentice Hall,Inc. Electrons in the first molecule The 5+end of the Ist momentarily becomes unevenly attracts the electrons in the distributed. 2nd and create a 6 end.• Interaction between two adjacent neutral molecules or atoms. 4. London Dispersion Forces Intermolecular Forces I (van der Waals forces) 伦敦色散力 The δ+ end of the 1st attracts the electrons in the 2nd and create a δ- end . Electrons in the first molecule momentarily becomes unevenly distributed