正在加载图片...
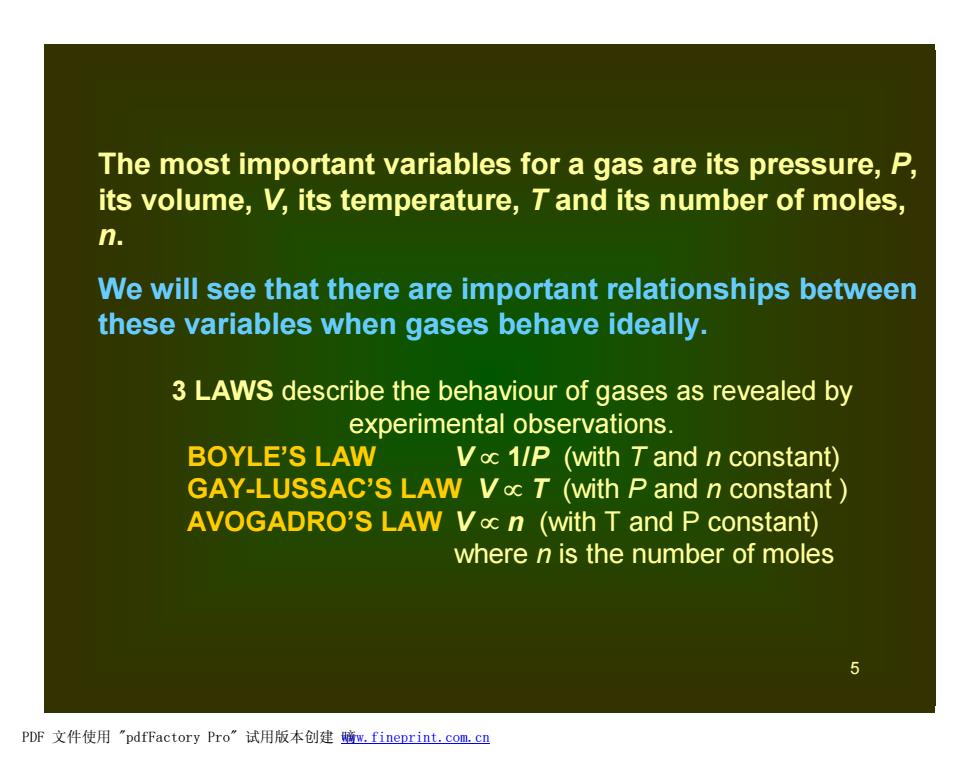
The most important variables for a gas are its pressure,P, its volume,V,its temperature,T and its number of moles, n. We will see that there are important relationships between these variables when gases behave ideally. 3 LAWS describe the behaviour of gases as revealed by experimental observations. BOYLE'S LAW Voc 1/P (with Tand n constant) GAY-LUSSAC'S LAW Vac T (with P and n constant AVOGADRO'S LAW Voc n (with T and P constant) where n is the number of moles PDF文件使用"pdfFactory Pro”试用版本创建暗m,fineprint.com,c里 5 The most important variables for a gas are its pressure, P, its volume, V, its temperature, T and its number of moles, n. We will see that there are important relationships between these variables when gases behave ideally. 3 LAWS describe the behaviour of gases as revealed by experimental observations. BOYLE’S LAW V µ 1/P (with T and n constant) GAY-LUSSAC’S LAW V µ T (with P and n constant ) AVOGADRO’S LAW V µ n (with T and P constant) where n is the number of moles PDF 文件使用 "pdfFactory Pro" 试用版本创建 嘀www.fineprint.com.cn