正在加载图片...
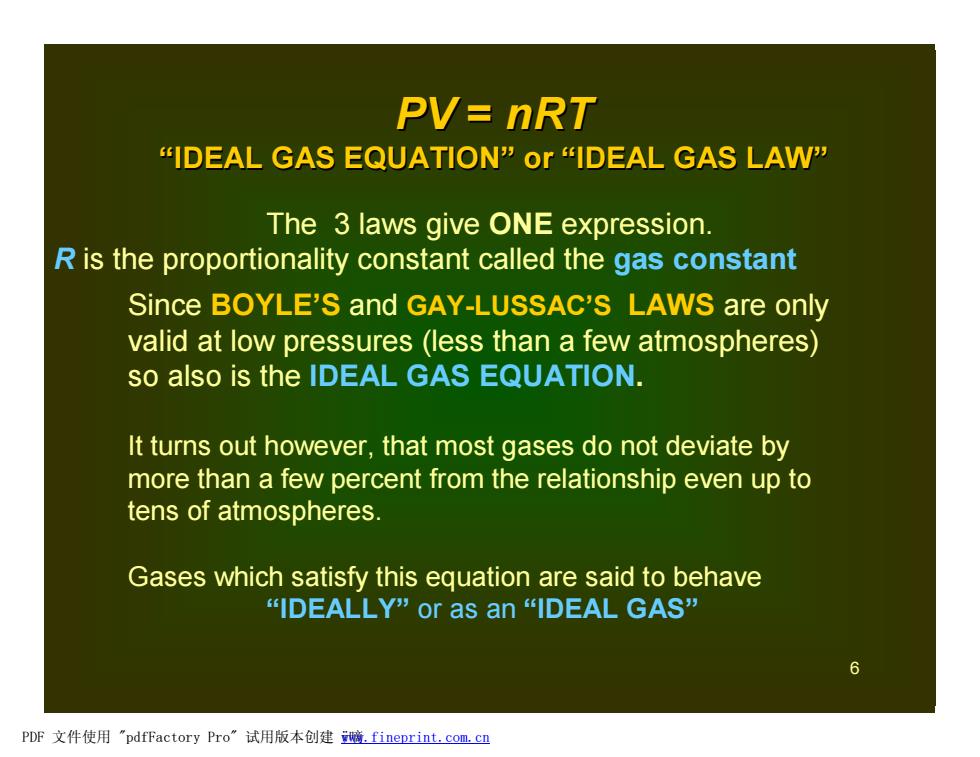
PV=nRT "IDEAL GAS EQUATION"or "IDEAL GAS LAW" The 3 laws give ONE expression. R is the proportionality constant called the gas constant Since BOYLE'S and GAY-LUSSAC'S LAWS are only valid at low pressures (less than a few atmospheres) so also is the IDEAL GAS EQUATION. It turns out however,that most gases do not deviate by more than a few percent from the relationship even up to tens of atmospheres. Gases which satisfy this equation are said to behave “IDEALLY"or as an“IDEAL GAS” PDF文件使用"pdfFactory Pro”试用版本创建脑.fineprint.com,cn6 The 3 laws give ONE expression. R is the proportionality constant called the gas constant PV = nRT “IDEAL GAS EQUATION” or “IDEAL GAS LAW” Since BOYLE’S and GAY-LUSSAC’S LAWS are only valid at low pressures (less than a few atmospheres) so also is the IDEAL GAS EQUATION. It turns out however, that most gases do not deviate by more than a few percent from the relationship even up to tens of atmospheres. Gases which satisfy this equation are said to behave “IDEALLY” or as an “IDEAL GAS” PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿ嘀www.fineprint.com.cn