正在加载图片...
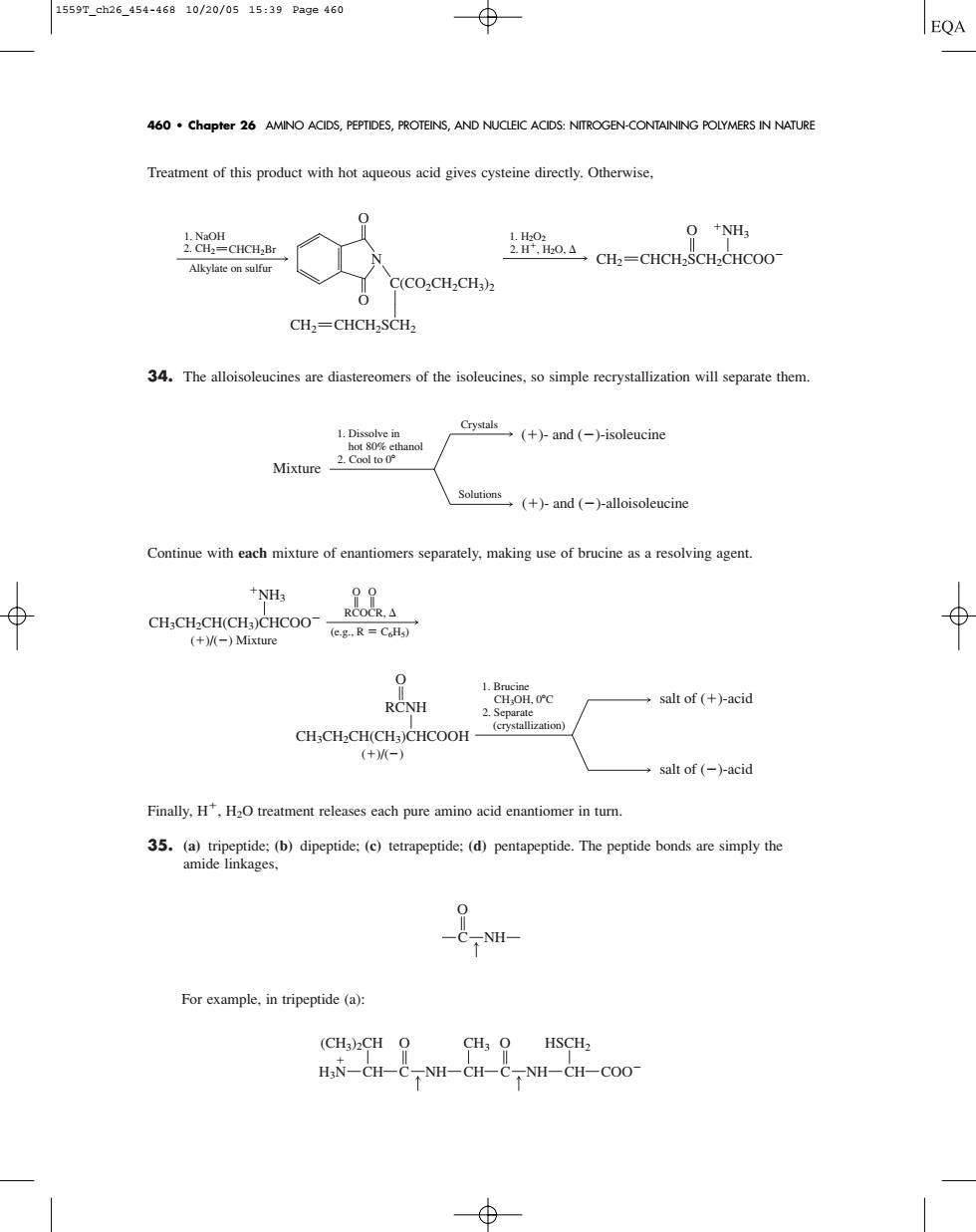
1559r.ah26.454-46910/20/0515:39Page460 460 chapter 26 AMINO ACIDS,PEPTIDES,PROTEINS,AND NUCLEI ACIDS:NITROGEN-CONTAINING POLYMERS IN NATURE Treament of this product with hotaqueous acid gives cysteine irectly.Otherwise. O +NH 上2odCH=CHCH SCH.CHCO0 C(CO.CH.CH) CH,=CHCH-SCH, 34.The alloisoleucines are diastereomers of the isoleucines.so simple recrystallization will separate them Crystals (+)-and(-)-isoleucine Mixture Continue with each mixture of enantiomers separately,making use of brucine as a resolving agent. NH3 (eg-R=) salt of (+)-acid +M salt of (-)-acid Finally,releases each pure amino acid enantiomer in tur. 35.The本er For example in tripeptide (a): (CH3)2CH O CH3 O HSCH2 HN-CH-C.NH-CH-C.NH-CH-COOTreatment of this product with hot aqueous acid gives cysteine directly. Otherwise, 34. The alloisoleucines are diastereomers of the isoleucines, so simple recrystallization will separate them. Continue with each mixture of enantiomers separately, making use of brucine as a resolving agent. Finally, H, H2O treatment releases each pure amino acid enantiomer in turn. 35. (a) tripeptide; (b) dipeptide; (c) tetrapeptide; (d) pentapeptide. The peptide bonds are simply the amide linkages, For example, in tripeptide (a): (CH3)2CH CH3 HSCH2 CH C NH CH COO CH C NH O O H3N C NH O 1. Brucine CH3OH, 0C 2. Separate (crystallization) salt of ()-acid salt of ()-acid CH3CH2CH(CH3)CHCOOH RCNH O ()/() NH3 CH3CH2CH(CH3)CHCOO ()/() Mixture RCOCR, O O (e.g., R C6H5) 1. Dissolve in hot 80% ethanol 2. Cool to 0 Solutions Crystals Mixture ()- and ()-isoleucine ()- and ()-alloisoleucine O O N CH2 CHCH2SCH2 C(CO2CH2CH3)2 1. NaOH Alkylate on sulfur 2. CH2 CHCH2Br 2. H, H2O, 1. H2O2 O NH3 CHCH2SCH2CHCOO CH2 460 • Chapter 26 AMINO ACIDS, PEPTIDES, PROTEINS, AND NUCLEIC ACIDS: NITROGEN-CONTAINING POLYMERS IN NATURE 1559T_ch26_454-468 10/20/05 15:39 Page 460������������������