正在加载图片...
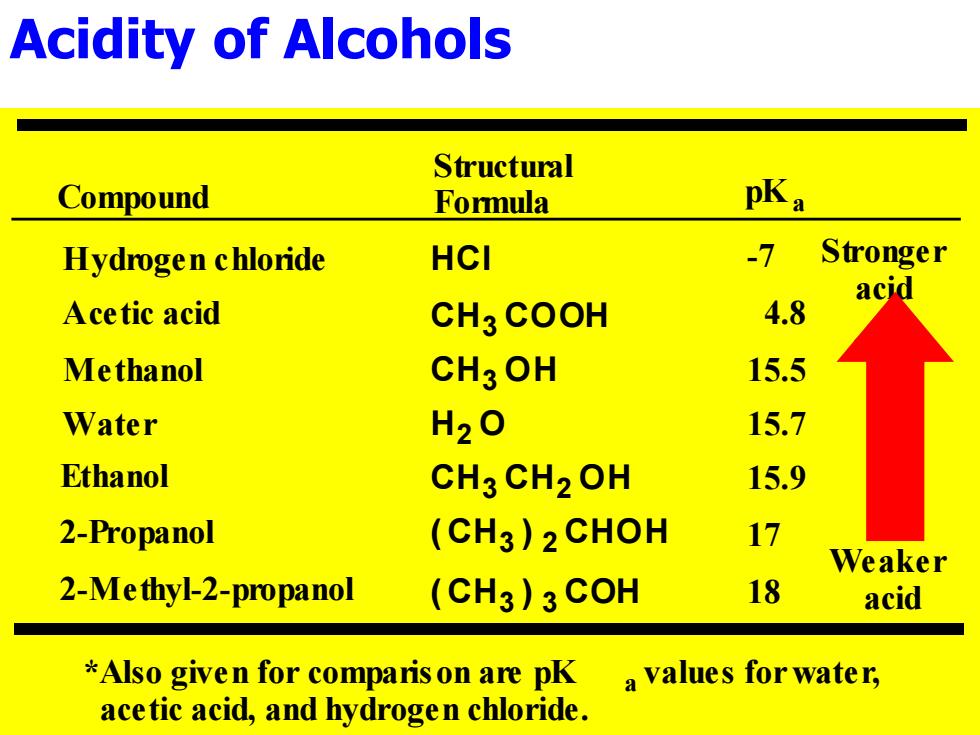
Acidity of Alcohols Structural Compound Formula pKa Hydrogen chloride HCI Stronger acid Acetic acid CH3 COOH 4.8 Methanol CH3OH 15.5 Water H2O 15.7 Ethanol CH3 CH2OH 15.9 2-Propanol (CH3)2CHOH Weaker 2-Me thyl-2-propanol (CH3)3COH 18 acid *Also given for comparis on are pK a values for water, acetic acid,and hydrogen chloride.Acidity of Alcohols ( CH3 ) 3 COH ( CH3 ) 2 CHOH CH3 CH2 OH H2 O CH3 OH CH3 COOH Hydrogen chloride HCl Acetic acid Methanol Water Ethanol 2-Propanol 2-Methyl-2-propanol Structural Formula Stronger acid Weaker acid *Also given for comparison are pK a values for water, acetic acid, and hydrogen chloride. Compound pKa -7 15.5 15.7 15.9 17 18 4.8