正在加载图片...
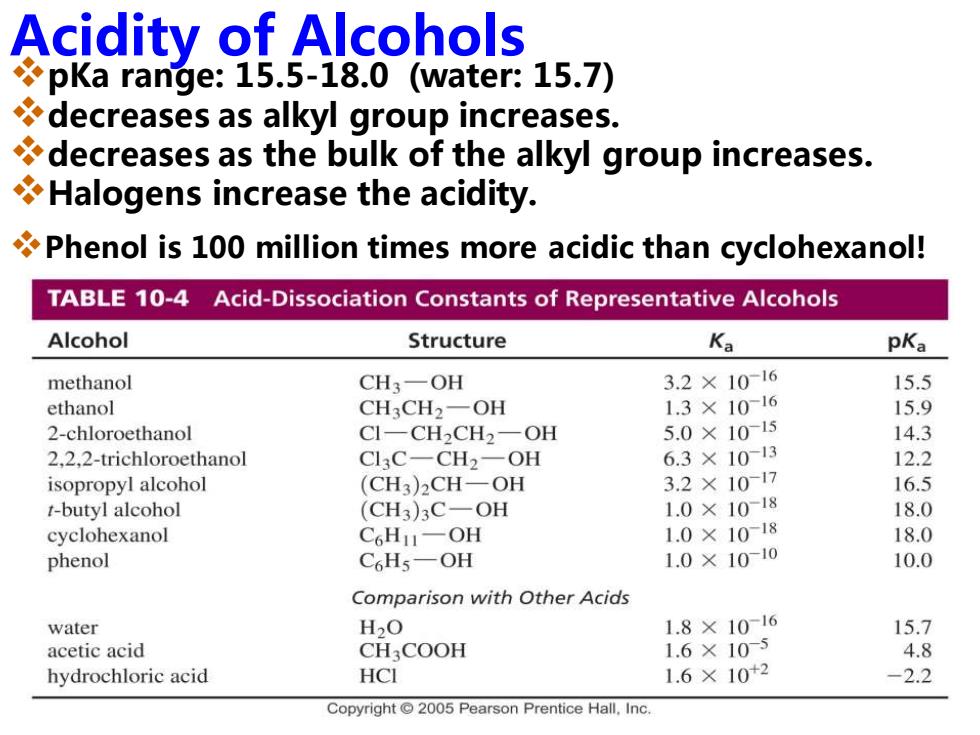
Acidity of Alcohols pKa range:15.5-18.0 (water:15.7) decreases as alkyl group increases. decreases as the bulk of the alkyl group increases. Halogens increase the acidity. Phenol is 100 million times more acidic than cyclohexanol! TABLE 10-4 Acid-Dissociation Constants of Representative Alcohols Alcohol Structure Ka pKa methanol CH3-OH 3.2×10-16 15.5 ethanol CH3CH2-OH 1.3×1016 15.9 2-chloroethanol C1-CH2CH2一OH 5.0×1015 14.3 2.2,2-trichloroethanol Cl3C-CH2-OH 6.3×1013 12.2 isopropyl alcohol (CH3)2CH-OH 3.2×10-17 16.5 t-butyl alcohol (CH3)3C-OH 1.0×1018 18.0 cyclohexanol C6H11一OH 1.0×1018 18.0 phenol C6H5-OH 1.0×1010 10.0 Comparison with Other Acids water H2O 1.8×10-16 15.7 acetic acid CH COOH 1.6×105 4.8 hydrochloric acid HCI 1.6×10+2 -2.2 Copyright 2005 Pearson Prentice Hall.Inc. Acidity of Alcohols ❖pKa range: 15.5-18.0 (water: 15.7) ❖decreases as alkyl group increases. ❖decreases as the bulk of the alkyl group increases. ❖Halogens increase the acidity. ❖Phenol is 100 million times more acidic than cyclohexanol!