正在加载图片...
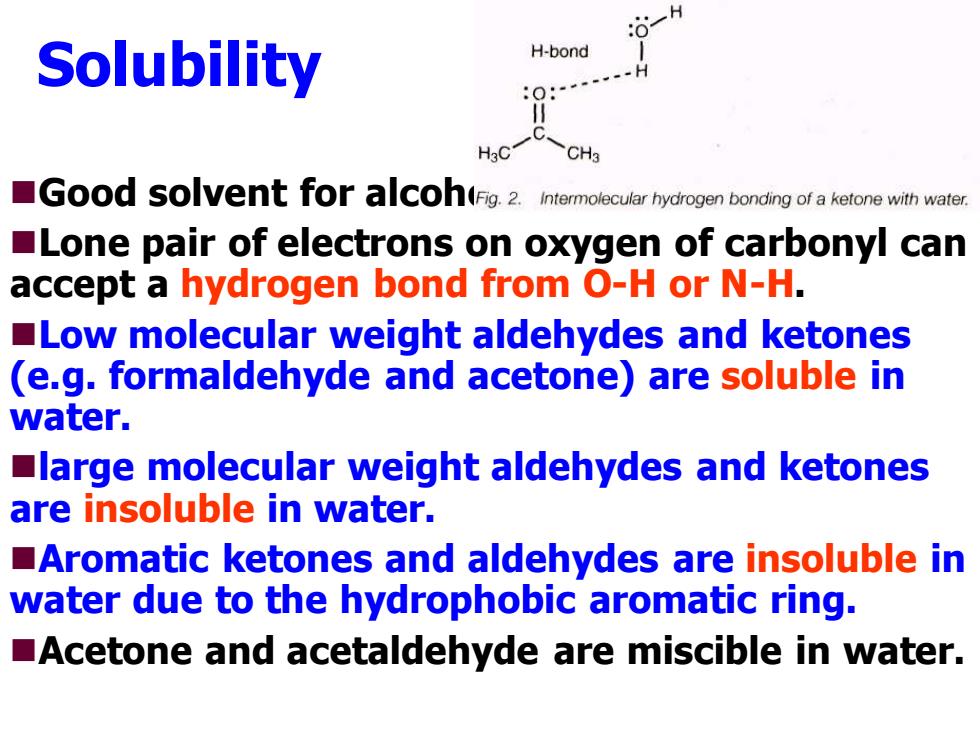
H Solubility H-bond H3C CH3 Good sovent foracoh(Fig.2.Intermolecular hydrogen bonding of a ketone with water. Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from O-H or N-H. Low molecular weight aldehydes and ketones (e.g.formaldehyde and acetone)are soluble in water. large molecular weight aldehydes and ketones are insoluble in water. Aromatic ketones and aldehydes are insoluble in water due to the hydrophobic aromatic ring. Acetone and acetaldehyde are miscible in water.Solubility ◼Good solvent for alcohols. ◼Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from O-H or N-H. ◼Low molecular weight aldehydes and ketones (e.g. formaldehyde and acetone) are soluble in water. ◼large molecular weight aldehydes and ketones are insoluble in water. ◼Aromatic ketones and aldehydes are insoluble in water due to the hydrophobic aromatic ring. ◼Acetone and acetaldehyde are miscible in water