正在加载图片...
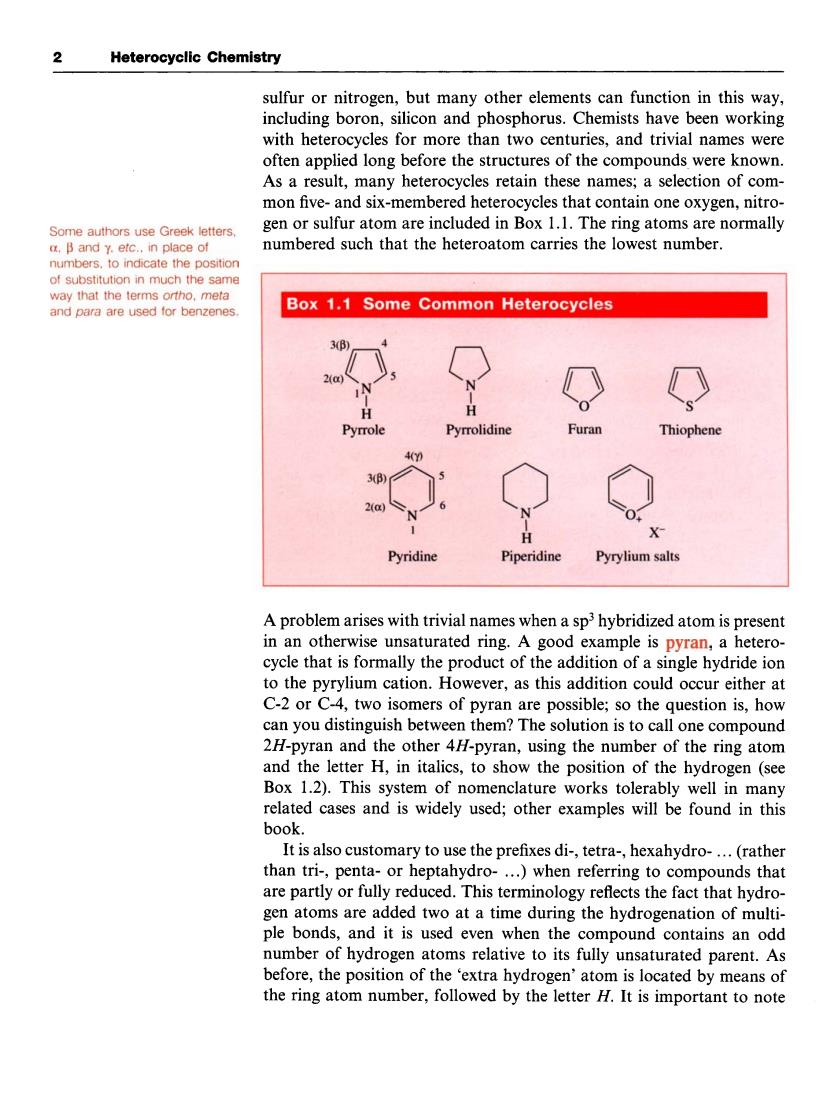
2 Heterocyclic Chemistry sulfurornitrogen,but many other elements can function in this way including boron,silicon and phosphorus.Chemists have been working with heterocycles for more than two centuries,and trivial names were often applied long before the structures of the compounds were known. As a result,many heterocycles retain these names;a selection of com- mon five-and six-membered heterocycles that contain one oxygen,nitro gen or sulfur atom are included in Box 1.1.The ring atoms are normally numbered such that the heteroatom carries the lowest number. of substitution in much the same Box 1.1 Some Common Heterocycles Pyrrole Pyrrolidine Furan Thiophene Pyridine Pyrylium salts A problem arises with trivial names when a sphybridized atom is present in an otherwise unsaturated ring.A good example is pyran,a hetero- cycle that is formally the product of the addition of a single hydride ion to the pyrylium cation.However,as this addition could occur either at C-2 or C-4,two isomers of pyrar are possible. so the question is,how can you distinguish between them?The solution is to call one compound 2H-pyran and the other 4//-pyran,using the number of the ring atom and the letter H,in italics,to show the position of the hydrogen (see Box 1.2).This tem of nom enclature works tolerably y well in many related cases and is widely used;other examples will be found in this book. It is also customary to use the prefixes di-,tetra-,hexahydro-...(rather than tri-,penta-or he eptahydro. …)when referring to omp nds that are partly or fully reduced.This terminology reflects the fact that hydro gen atoms are added two at a time during the hydrogenation of multi- ple bonds,and it is used even when the compound contains an odd number of hydrogen atoms relative to its fully unsaturated parent.As before,the position of the 'extra hydrogen'atom is located by means of the ring atom number,followed by the letter H.It is important to note 2 Heterocyclic Chemistry sulfur or nitrogen, but many other elements can function in this way, including boron, silicon and phosphorus. Chemists have been working with heterocycles for more than two centuries, and trivial names were often applied long before the structures of the compounds were known. As a result, many heterocycles retain these names; a selection of common five- and six-membered heterocycles that contain one oxygen, nitrogen or sulfur atom are included in Box 1.1. The ring atoms are normally numbered such that the heteroatom carries the lowest number. Some authors use Greek letters, a, p and y, etc., in place of numbers, to indicate the position of substitution in much the same way that the terms ortho, meta and para are used for benzenes. A problem arises with trivial names when a sp3 hybridized atom is present in an otherwise unsaturated ring. A good example is pyran, a heterocycle that is formally the product of the addition of a single hydride ion to the pyrylium cation. However, as this addition could occur either at C-2 or C-4, two isomers of pyran are possible; so the question is, how can you distinguish between them? The solution is to call one compound 2H-pyran and the other 4H-pyran, using the number of the ring atom and the letter H, in italics, to show the position of the hydrogen (see Box 1.2). This system of nomenclature works tolerably well in many related cases and is widely used; other examples will be found in this book. It is also customary to use the prefixes di-, tetra-, hexahydro- . . . (rather than tri-, penta- or heptahydro- ...) when referring to compounds that are partly or fully reduced. This terminology reflects the fact that hydrogen atoms are added two at a time during the hydrogenation of multiple bonds, and it is used even when the compound contains an odd number of hydrogen atoms relative to its fully unsaturated parent. As before, the position of the ‘extra hydrogen’ atom is located by means of the ring atom number, followed by the letter H. It is important to note