正在加载图片...
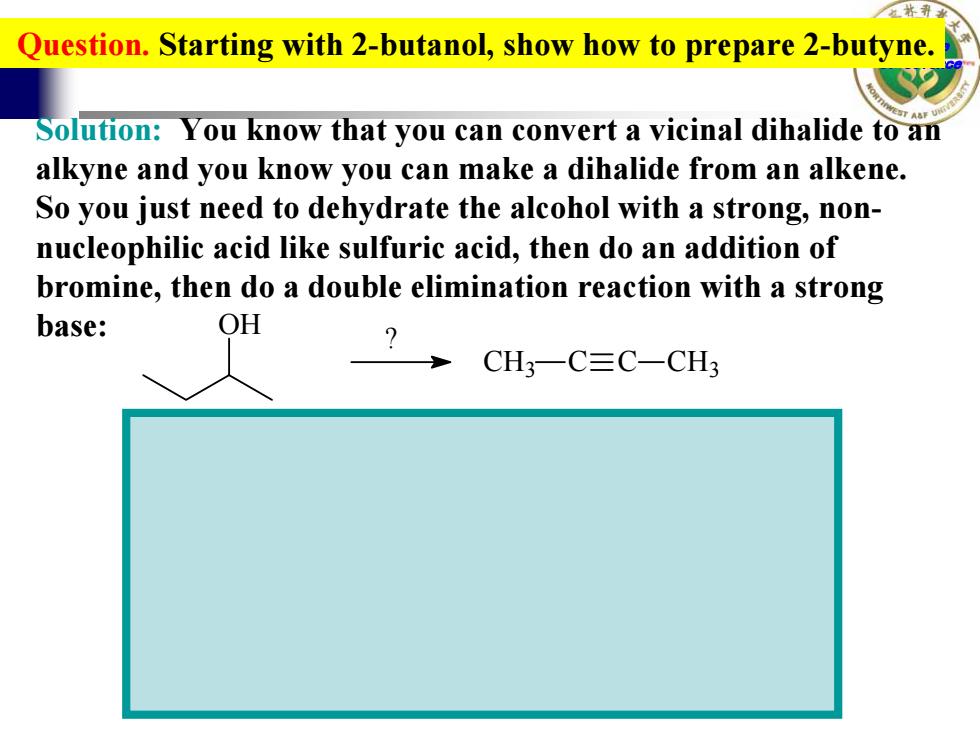
Question.Starting with 2-butanol,show how to prepare 2-butyne. Solution:You know that you can convert a vicinal dihalide to an alkyne and you know you can make a dihalide from an alkene. So you just need to dehydrate the alcohol with a strong,non- nucleophilic acid like sulfuric acid,then do an addition of bromine,then do a double elimination reaction with a strong base: OH CH3一C≡C-CH3 College of Science Question. Starting with 2-butanol, show how to prepare 2-butyne. OH CH3 C C CH3 ? H2SO4, heat Br2 CCl4 Br Br 2 equivalents NaNH2 Solution: You know that you can convert a vicinal dihalide to an alkyne and you know you can make a dihalide from an alkene. So you just need to dehydrate the alcohol with a strong, nonnucleophilic acid like sulfuric acid, then do an addition of bromine, then do a double elimination reaction with a strong base: