正在加载图片...
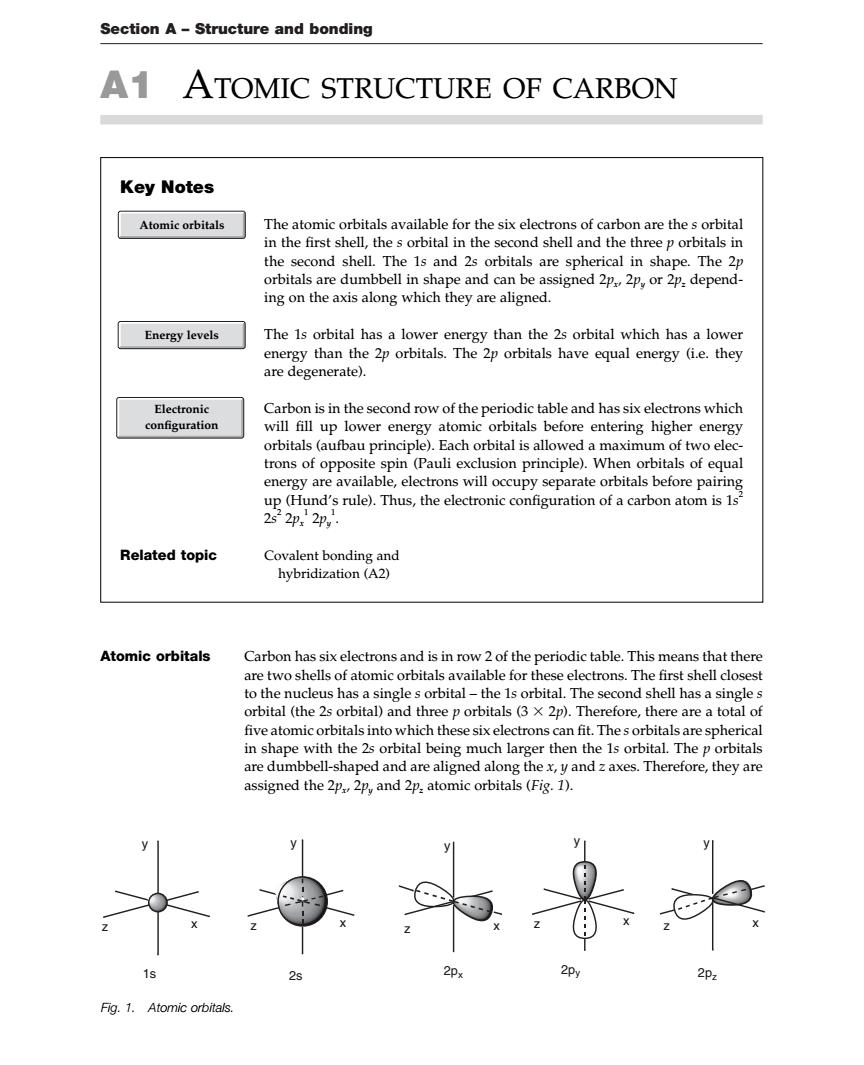
Section A-Structure and bonding A1 ATOMIC STRUCTURE OF CARBON Key Notes Atomic orbitals ,thesorbital shel orbitals are 2公金aee马 hape and can be assigned 2p2p,or 2p.depend- ing on the axis along which they are aligned. Energy levels The 1s orbital has a lower energy than the 2s orbital which has a lower energy than the 2p orbitals.The 2p orbitals have equal energy (i.e.they are degenerate). Electronic Carbon is in the second row of the periodic table and has six electrons which configuration will fill up lower energy atomic orbitals before entering higher energy orbitals(aufbau principle).Each orbital is allowed a maximum of two elec trons of opposite spin (Pauli exclusion principle).When orbitals of equal energy are available,electrons will occupy separate orbitals before pairing 2器2m.n,感he Related topic Covalent bonding and hybridization(A2) Atomic orbitals Carbon has six electrons and is in row 2 of the periodic table.This means that there are two shells of atomic orbitals available for these electrons The first shell closest to the nucleus has a single s orbital-the 1s orbital.The second shell has a single s orbital (the 2s orbital)and three p orbitals (3 x 2p).Therefore,there are a total of five atomic orbitals into which these six electrons can fit thes orbitals an in shape with the 2s orbital being much large are dumbbell-shaped and are aligned along the x,yand z axes.Therefore,they are ssigned the and patomic orbitals (Fig.1). 4是@ Fig.1.Atomic orbitals Section A – Structure and bonding A1 ATOMIC STRUCTURE OF CARBON Atomic orbitals Carbon has six electrons and is in row 2 of the periodic table. This means that there are two shells of atomic orbitals available for these electrons. The first shell closest to the nucleus has a single s orbital – the 1s orbital. The second shell has a single s orbital (the 2s orbital) and three p orbitals (3 2p). Therefore, there are a total of five atomic orbitals into which these six electrons can fit. The s orbitals are spherical in shape with the 2s orbital being much larger then the 1s orbital. The p orbitals are dumbbell-shaped and are aligned along the x, y and z axes. Therefore, they are assigned the 2px, 2py and 2pz atomic orbitals (Fig. 1). Key Notes The atomic orbitals available for the six electrons of carbon are the s orbital in the first shell, the s orbital in the second shell and the three p orbitals in the second shell. The 1s and 2s orbitals are spherical in shape. The 2p orbitals are dumbbell in shape and can be assigned 2px, 2py or 2pz depending on the axis along which they are aligned. The 1s orbital has a lower energy than the 2s orbital which has a lower energy than the 2p orbitals. The 2p orbitals have equal energy (i.e. they are degenerate). Carbon is in the second row of the periodic table and has six electrons which will fill up lower energy atomic orbitals before entering higher energy orbitals (aufbau principle). Each orbital is allowed a maximum of two electrons of opposite spin (Pauli exclusion principle). When orbitals of equal energy are available, electrons will occupy separate orbitals before pairing up (Hund’s rule). Thus, the electronic configuration of a carbon atom is 1s 2 2s 2 2px 1 2py 1 . Related topic Covalent bonding and hybridization (A2) Electronic configuration Atomic orbitals Energy levels 1s 2s 2px 2py 2pz y z x y z x y z x y z x y z x Fig. 1. Atomic orbitals