正在加载图片...
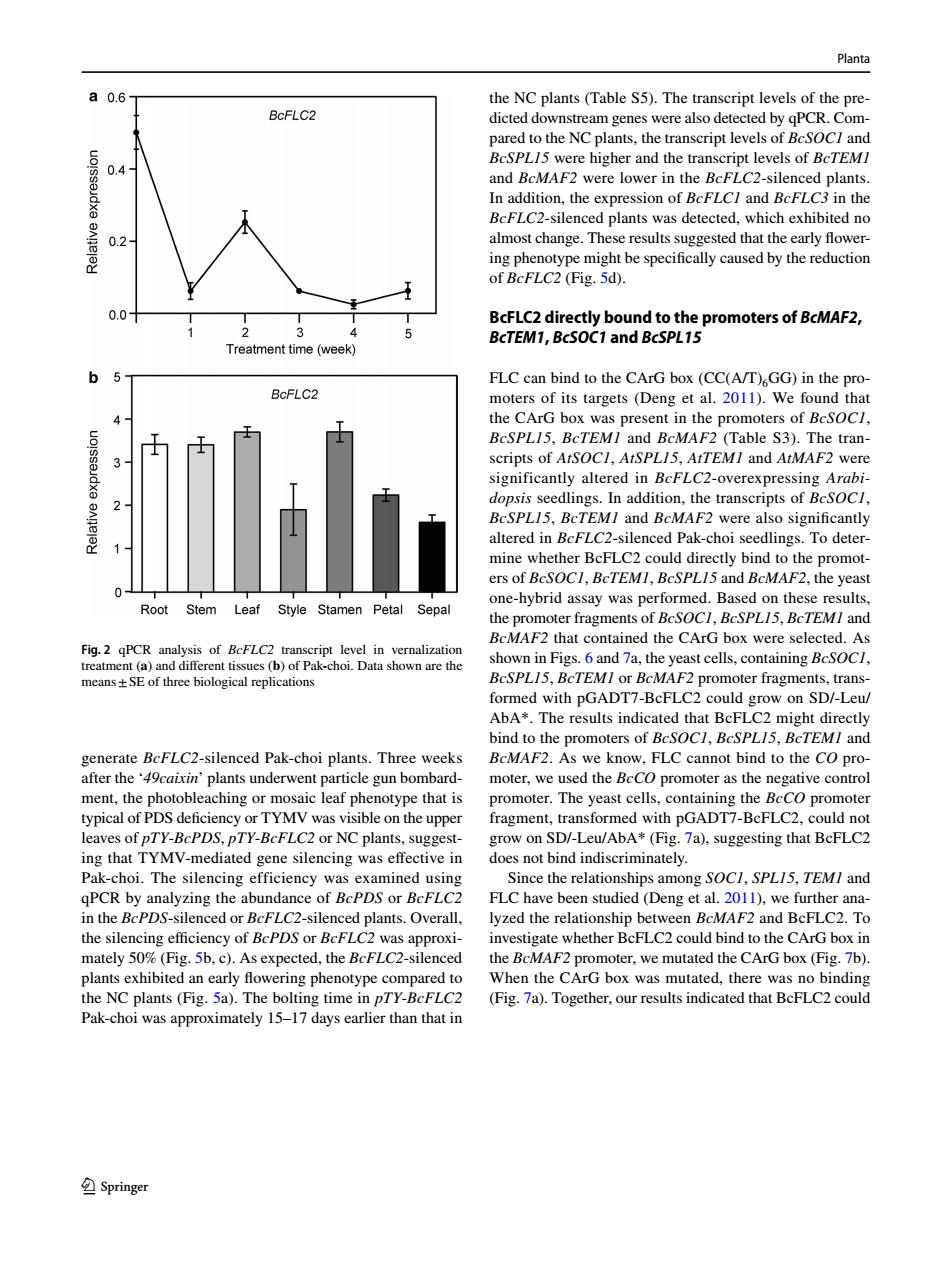
Planta 0.6 the NC plants(Table S5).The transcript levels of the pre- BcFLC2 dicted downstream genes were also detected by qPCR.Com- pared to the NC plants,the transcript levels of BcSOC/and BcSPL15 were higher and the transcript levels of BcTEMI 0.4 and BcMAF2 were lower in the BcFLC2-silenced plants. In addition,the expression of BcFLCI and BcFLC3 in the BcFLC2-silenced plants was detected,which exhibited no 0.2 almost change.These results suggested that the early flower- ing phenotype might be specifically caused by the reduction of BcFLC2(Fig.5d). 0.0 BcFLC2 directly bound to the promoters of BcMAF2, 2 BcTEM1,BcSOC1 and BcSPL15 Treatment time (week) 6 FLC can bind to the CArG box(CC(A/T)GG)in the pro- BcFLC2 moters of its targets (Deng et al.2011).We found that the CArG box was present in the promoters of BcSOC/, BcSPL15,BcTEMI and BcMAF2 (Table S3).The tran- scripts of AtSOCl,AtSPL15,AtTEMI and AtMAF2 were significantly altered in BcFLC2-overexpressing Arabi- dopsis seedlings.In addition,the transcripts of BcSOCI, BcSPL15,BcTEMI and BcMAF2 were also significantly altered in BcFLC2-silenced Pak-choi seedlings.To deter- mine whether BcFLC2 could directly bind to the promot- ers of BcSOC1,BcTEMI,BcSPL15 and BcMAF2,the yeast one-hybrid assay was performed.Based on these results, Root Stem Leaf Style Stamen Petal Sepal the promoter fragments of BcSOCI,BcSPL15,BcTEMI and BcMAF2 that contained the CArG box were selected.As Fig.2 gPCR analysis of BcFLC2 transcript level in vernalization treatment(a)and different tissues(b)of Pak-choi.Data shown are the shown in Figs.6 and 7a,the yeast cells.containing BcSOC/. means+SE of three biological replications BcSPL15,BcTEMI or BcMAF2 promoter fragments,trans- formed with pGADT7-BcFLC2 could grow on SD/-Leu/ AbA*.The results indicated that BcFLC2 might directly bind to the promoters of BcSOCI,BcSPL/5,BcTEMI and generate BcFLC2-silenced Pak-choi plants.Three weeks BCMAF2.As we know,FLC cannot bind to the CO pro- after the '49caixin'plants underwent particle gun bombard- moter,we used the BcCO promoter as the negative control ment,the photobleaching or mosaic leaf phenotype that is promoter.The yeast cells,containing the BcCO promoter typical of PDS deficiency or TYMV was visible on the upper fragment,transformed with pGADT7-BcFLC2,could not leaves of pTY-BcPDS,pTY-BcFLC2 or NC plants,suggest- grow on SD/-Leu/AbA*(Fig.7a),suggesting that BcFLC2 ing that TYMV-mediated gene silencing was effective in does not bind indiscriminately. Pak-choi.The silencing efficiency was examined using Since the relationships among SOCI,SPL15,TEMI and qPCR by analyzing the abundance of BcPDS or BcFLC2 FLC have been studied(Deng et al.2011),we further ana- in the BcPDS-silenced or BcFLC2-silenced plants.Overall. lyzed the relationship between BcMAF2 and BcFLC2.To the silencing efficiency of BcPDS or BcFLC2 was approxi- investigate whether BcFLC2 could bind to the CArG box in mately 50%(Fig.5b,c).As expected,the BcFLC2-silenced the BcMAF2 promoter,we mutated the CArG box(Fig.7b). plants exhibited an early flowering phenotype compared to When the CArG box was mutated,there was no binding the NC plants (Fig.5a).The bolting time in pTY-BcFLC2 (Fig.7a).Together,our results indicated that BcFLC2 could Pak-choi was approximately 15-17 days earlier than that in SpringerPlanta 1 3 generate BcFLC2-silenced Pak-choi plants. Three weeks after the ‘49caixin’ plants underwent particle gun bombardment, the photobleaching or mosaic leaf phenotype that is typical of PDS defciency or TYMV was visible on the upper leaves of pTY-BcPDS, pTY-BcFLC2 or NC plants, suggesting that TYMV-mediated gene silencing was efective in Pak-choi. The silencing efficiency was examined using qPCR by analyzing the abundance of BcPDS or BcFLC2 in the BcPDS-silenced or BcFLC2-silenced plants. Overall, the silencing efciency of BcPDS or BcFLC2 was approximately 50% (Fig. 5b, c). As expected, the BcFLC2-silenced plants exhibited an early fowering phenotype compared to the NC plants (Fig. 5a). The bolting time in pTY-BcFLC2 Pak-choi was approximately 15–17 days earlier than that in the NC plants (Table S5). The transcript levels of the predicted downstream genes were also detected by qPCR. Compared to the NC plants, the transcript levels of BcSOC1 and BcSPL15 were higher and the transcript levels of BcTEM1 and BcMAF2 were lower in the BcFLC2-silenced plants. In addition, the expression of BcFLC1 and BcFLC3 in the BcFLC2-silenced plants was detected, which exhibited no almost change. These results suggested that the early fowering phenotype might be specifcally caused by the reduction of BcFLC2 (Fig. 5d). BcFLC2 directly bound to the promoters of BcMAF2, BcTEM1, BcSOC1 and BcSPL15 FLC can bind to the CArG box (CC(A/T)6GG) in the promoters of its targets (Deng et al. 2011). We found that the CArG box was present in the promoters of BcSOC1, BcSPL15, BcTEM1 and BcMAF2 (Table S3). The transcripts of AtSOC1, AtSPL15, AtTEM1 and AtMAF2 were significantly altered in BcFLC2-overexpressing Arabidopsis seedlings. In addition, the transcripts of BcSOC1, BcSPL15, BcTEM1 and BcMAF2 were also signifcantly altered in BcFLC2-silenced Pak-choi seedlings. To determine whether BcFLC2 could directly bind to the promoters of BcSOC1, BcTEM1, BcSPL15 and BcMAF2, the yeast one-hybrid assay was performed. Based on these results, the promoter fragments of BcSOC1, BcSPL15, BcTEM1 and BcMAF2 that contained the CArG box were selected. As shown in Figs. 6 and 7a, the yeast cells, containing BcSOC1, BcSPL15, BcTEM1 or BcMAF2 promoter fragments, transformed with pGADT7-BcFLC2 could grow on SD/-Leu/ AbA*. The results indicated that BcFLC2 might directly bind to the promoters of BcSOC1, BcSPL15, BcTEM1 and BcMAF2. As we know, FLC cannot bind to the CO promoter, we used the BcCO promoter as the negative control promoter. The yeast cells, containing the BcCO promoter fragment, transformed with pGADT7-BcFLC2, could not grow on SD/-Leu/AbA* (Fig. 7a), suggesting that BcFLC2 does not bind indiscriminately. Since the relationships among SOC1, SPL15, TEM1 and FLC have been studied (Deng et al. 2011), we further analyzed the relationship between BcMAF2 and BcFLC2. To investigate whether BcFLC2 could bind to the CArG box in the BcMAF2 promoter, we mutated the CArG box (Fig. 7b). When the CArG box was mutated, there was no binding (Fig. 7a). Together, our results indicated that BcFLC2 could Fig. 2 qPCR analysis of BcFLC2 transcript level in vernalization treatment (a) and diferent tissues (b) of Pak-choi. Data shown are the means±SE of three biological replications