正在加载图片...
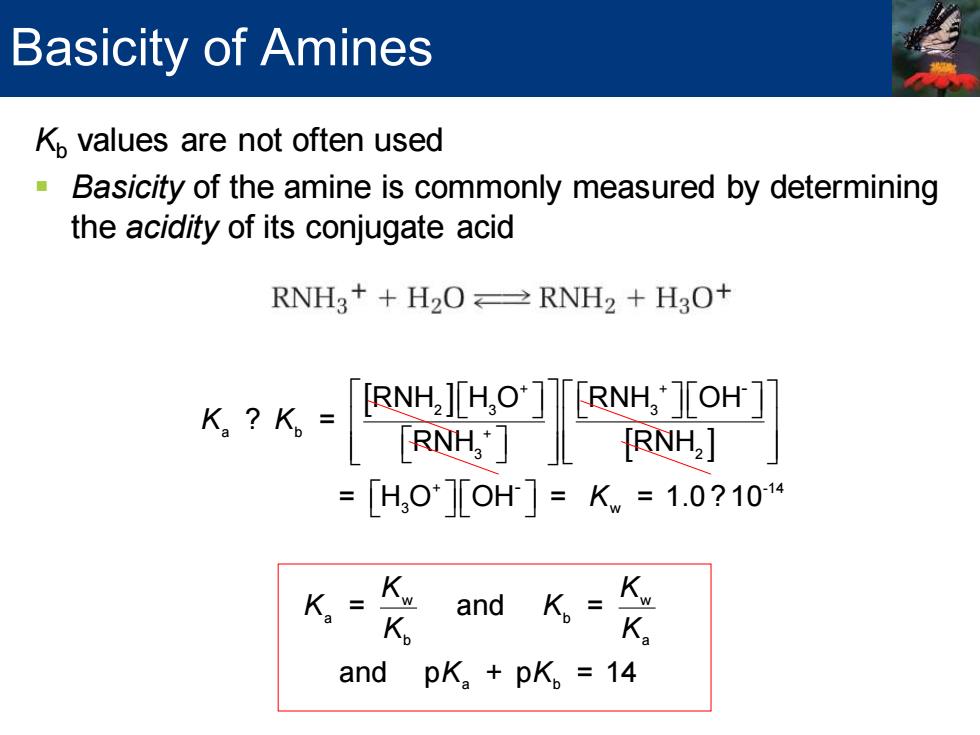
Basicity of Amines Kp values are not often used Basicity of the amine is commonly measured by determining the acidity of its conjugate acid RNHg++H2O≌RNH2+H3O+ 6k-90鬥 =[H,0][OH]=Kw=1.0?104 K= K and K.=K. K and pK.+pK。=14 Kb values are not often used ▪ Basicity of the amine is commonly measured by determining the acidity of its conjugate acid + + 3 2 2 3 + + 2 3 3 a b + 3 2 + -14 3 w w a b - - RNH + H O RNH + H O RNH H O RNH OH ? = RNH RNH = H O OH = = 1.0 ? 10 = K K K K K K w b a a b and = and p + p = 14 K K K K K Basicity of Amines