正在加载图片...
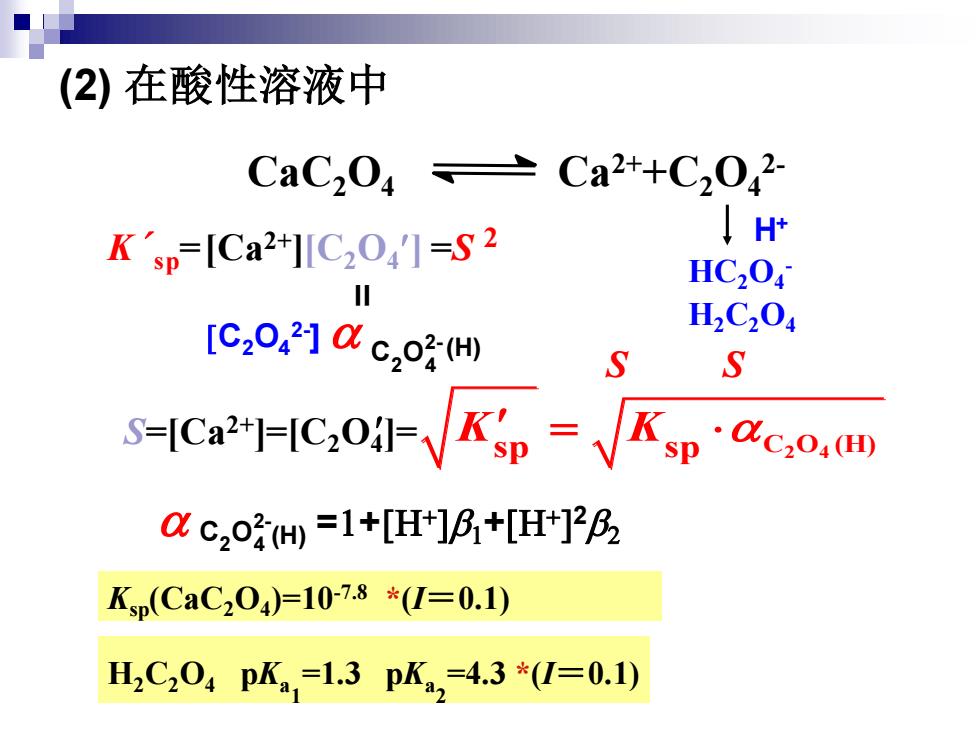
(2)在酸性溶液中 CaC204≥ Ca2+C2042 Ksp=Ca2+][C2041=S2 H+ HC,04 [C2042]c,oH H2C204 S S S=Ica2=C,0√K5p=√Kp c2o=1+[H]B+[Ht]2B2 Kp(CaC204)=10-7.8*(I=0.1) H2C204pK,=1.3pK,=4.3*(I=0.1)(2) 在酸性溶液中 CaC2O4 Ca2++C2O4 2- S S H+ Ksp(CaC2O4 )=10-7.8 *(I=0.1) H2C2O4 pKa 1 =1.3 pKa 2 =4.3 *(I=0.1) K´ sp =[Ca2+][C2O4 ] =S 2 = [C2O4 2- ] (H) 2- 2 4 C O (H) =1+[H+ ]b1+[H+ ] 2b2 2- 2 4 C O HC2O4 - H2C2O4 S=[Ca2+]=[C2O4 ]= sp sp C O (H) 2 4 K K =