正在加载图片...
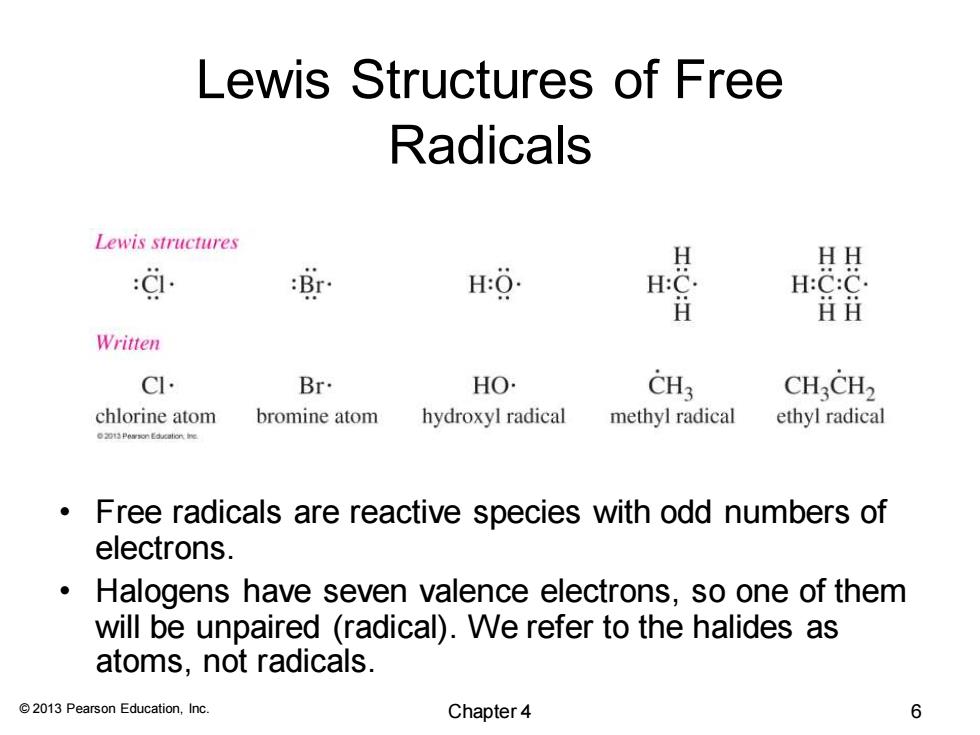
Lewis Structures of Free Radicals Lewis structures 效 H HH H H:C:C ⅱ丑 Written CI. Br. HO. CH3 CHCH2 chlorine atom bromine atom hydroxyl radical methyl radical ethyl radical Free radicals are reactive species with odd numbers of electrons. Halogens have seven valence electrons,so one of them will be unpaired (radical).We refer to the halides as atoms,not radicals. 2013 Pearson Education,Inc. Chapter 4 6 © 2013 Pearson Education, Inc. Lewis Structures of Free Radicals • Free radicals are reactive species with odd numbers of electrons. • Halogens have seven valence electrons, so one of them will be unpaired (radical). We refer to the halides as atoms, not radicals. Chapter 4 6