正在加载图片...
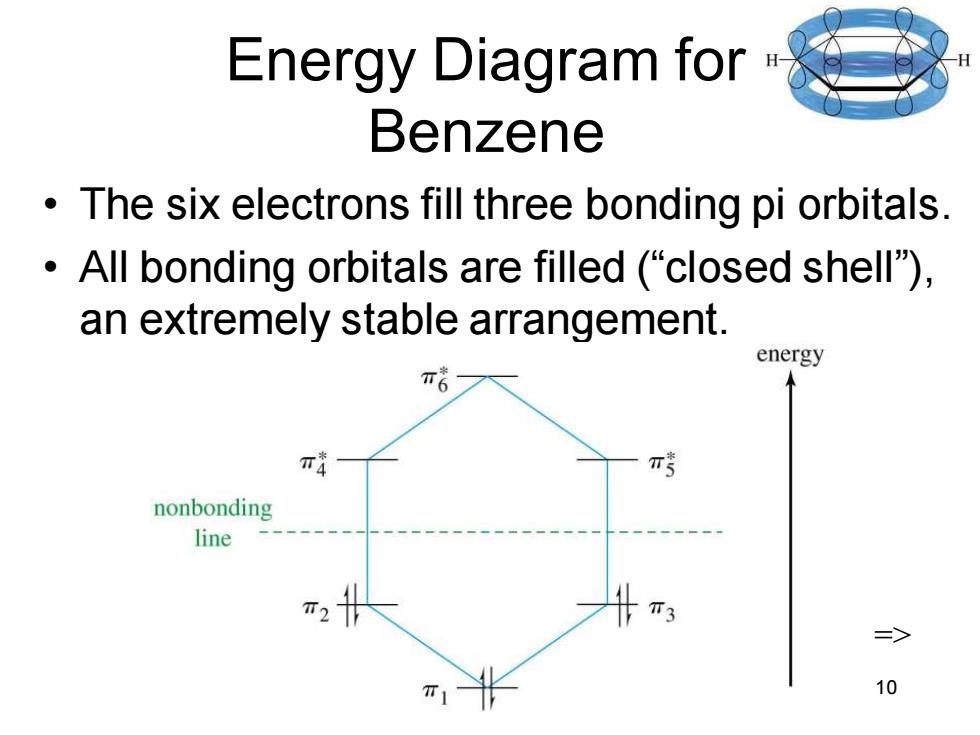
Energy Diagram for Benzene The six electrons fill three bonding pi orbitals. All bonding orbitals are filled ("closed shell). an extremely stable arrangement. energy π nonbonding line T3 10 Chapter 16 10 Energy Diagram for Benzene • The six electrons fill three bonding pi orbitals. • All bonding orbitals are filled (“closed shell”), an extremely stable arrangement. =>