正在加载图片...
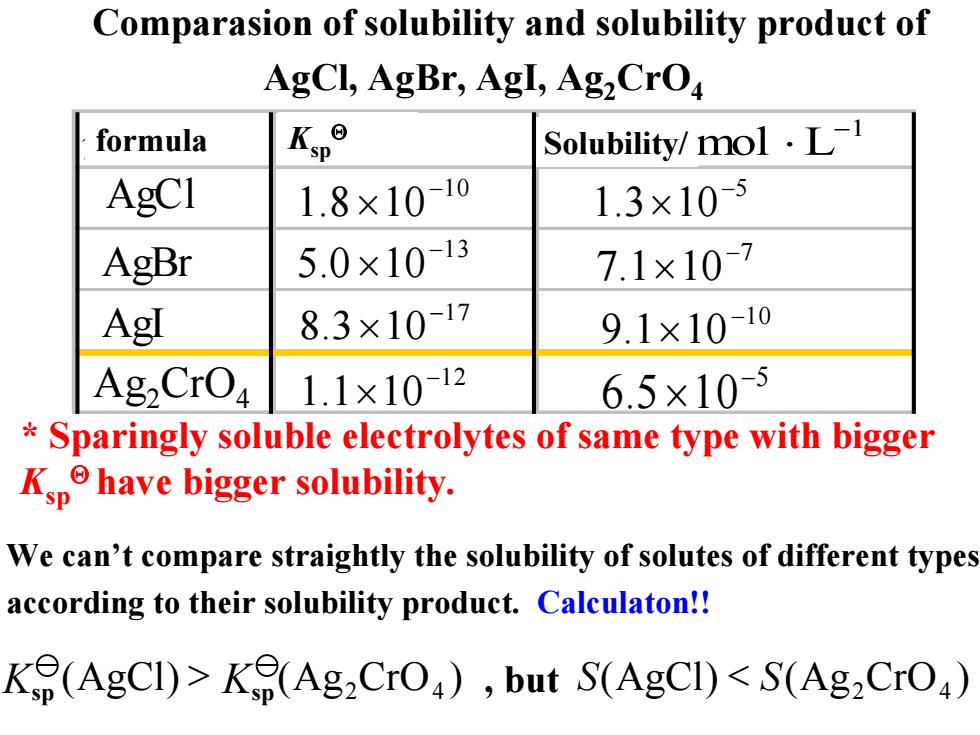
Comparasion of solubility and solubility product of AgCl,AgBr,Agl,Ag,CrO formula Kype Solubility/mol.L-1 AgCI 1.8×10-10 1.3×105 AgBr 5.0×1013 7.1×107 AgI 8.3×10-17 9.1×10-10 Ag2CrO 1.1×1012 6.5×10-5 Sparingly soluble electrolytes of same type with bigger Kphave bigger solubility. We can't compare straightly the solubility of solutes of different types according to their solubility product.Calculaton!! K(AgCI)>K(Ag2CrO),but S(AgCI)<S(Ag2CrO) (AgCl < SS () Ag 42 )CrO We can’t compare straightly the solubility of solutes of different types according to their solubility product. Calculaton!! * Sparingly soluble electrolytes of same type with bigger KspΘ have bigger solubility. Ksp (AgCl > Ksp () Ag 42 )CrO Comparasion of solubility and solubility product of AgCl, AgBr, AgI, Ag2CrO4 , but 分子式 溶度积 溶解度/ AgBr AgI AgCl 5 5.6 10− × 1 mol L− ⋅ 10 8.1 10 − × 13 0.5 10 − × 17 3.8 10− × 12 1.1 10− × 10 1.9 10 − × 7 1.7 10 − × 5 3.1 10− × Ag2CrO4 formula Ksp Solubility/ Θ