正在加载图片...
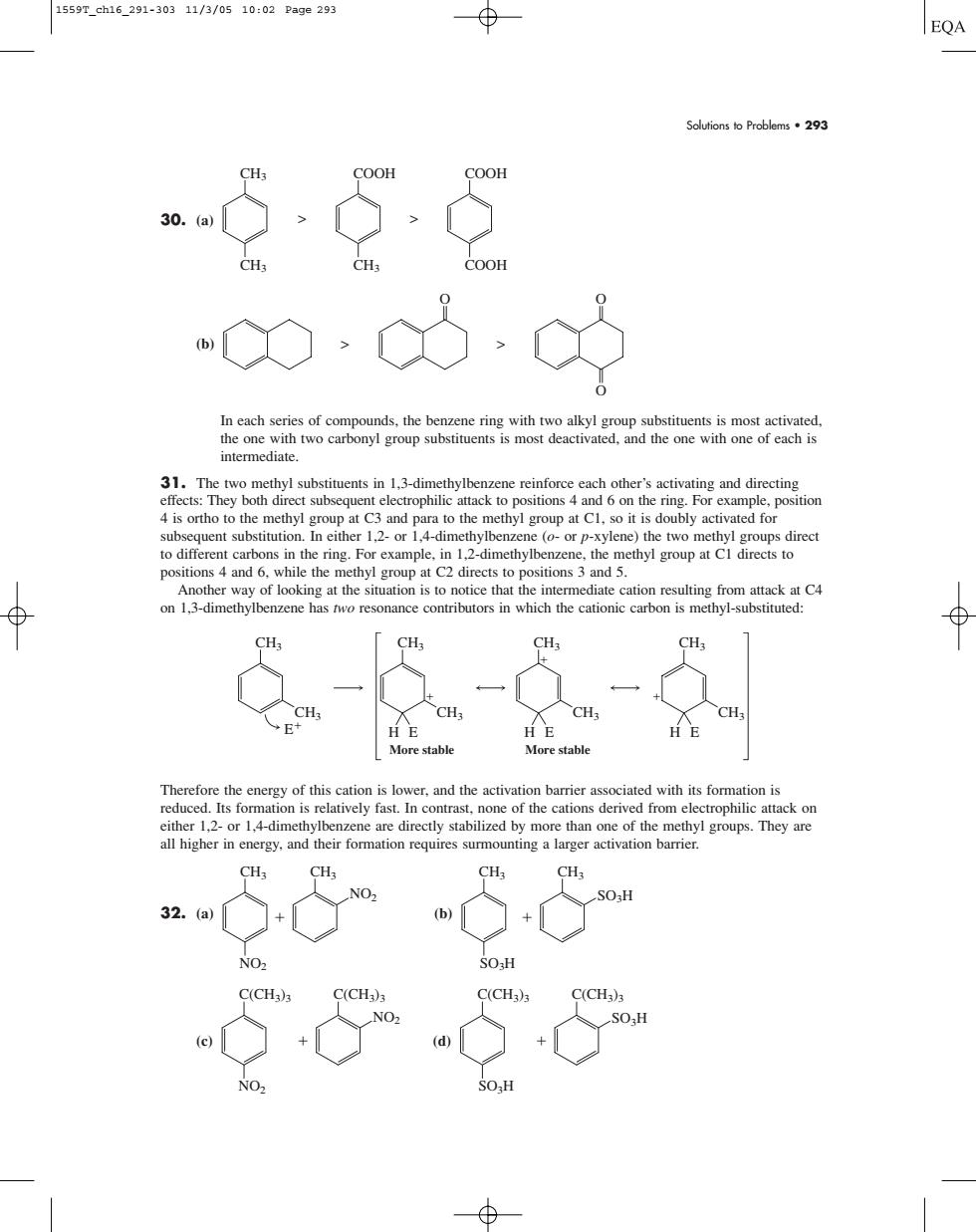
1559r.ah16.291-30311/3/0510:02Page293 EQA Soutions to Problems293 COOH COOH In each series of cor unde the hen e rina ted the onewith wo carbonyl group substituentsis most deactivated.and the of eachis intermediate. 31.The two ethyl substituents in 1 3-din other' ubly activated fo to different carbon n theing For 1-dimethy benzene.the methyl positions 4 and 6. while the methyl group at C2 directs to positions 3 and 5 H More stah More ociatedwithisfopiliCn either 12-or 14-dimethyibenze e are directly stabilized by more than one of the methyl groups.They are all higher in energy,and their formation requires surmounting a larger activation barrier CH CH 32.a) + b)1 + SO-H CHSolutions to Problems • 293 30. (a) (b) In each series of compounds, the benzene ring with two alkyl group substituents is most activated, the one with two carbonyl group substituents is most deactivated, and the one with one of each is intermediate. 31. The two methyl substituents in 1,3-dimethylbenzene reinforce each other’s activating and directing effects: They both direct subsequent electrophilic attack to positions 4 and 6 on the ring. For example, position 4 is ortho to the methyl group at C3 and para to the methyl group at C1, so it is doubly activated for subsequent substitution. In either 1,2- or 1,4-dimethylbenzene (o- or p-xylene) the two methyl groups direct to different carbons in the ring. For example, in 1,2-dimethylbenzene, the methyl group at C1 directs to positions 4 and 6, while the methyl group at C2 directs to positions 3 and 5. Another way of looking at the situation is to notice that the intermediate cation resulting from attack at C4 on 1,3-dimethylbenzene has two resonance contributors in which the cationic carbon is methyl-substituted: Therefore the energy of this cation is lower, and the activation barrier associated with its formation is reduced. Its formation is relatively fast. In contrast, none of the cations derived from electrophilic attack on either 1,2- or 1,4-dimethylbenzene are directly stabilized by more than one of the methyl groups. They are all higher in energy, and their formation requires surmounting a larger activation barrier. 32. (a) (b) (c) (d) C(CH3)3 C(CH3)3 SO3H SO3H C(CH3)3 C(CH3)3 NO2 NO2 CH3 SO3H CH3 SO3H CH3 NO2 CH3 NO2 CH3 CH3 E H CH3 CH3 E CH3 CH3 H E CH3 CH3 H E More stable More stable > > O O O CH3 CH3 > COOH CH3 > COOH COOH 1559T_ch16_291-303 11/3/05 10:02 Page 293��������