正在加载图片...
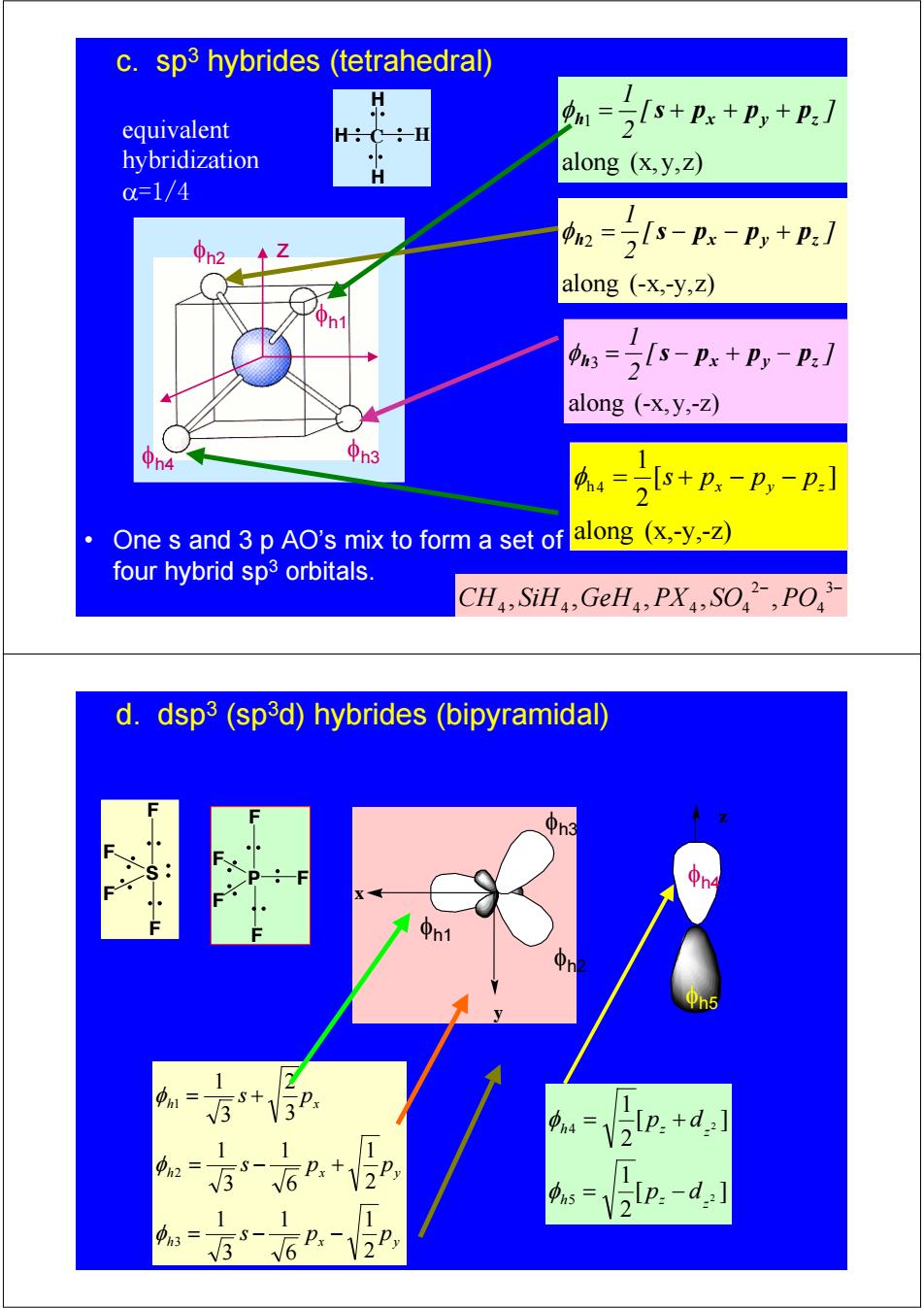
c.sp3 hybrides (tetrahedral) equivalent 1= 2+px+p,+P:] -H hybridization H along (x,y,z) c=1/4 2 22s-n-p,+:/ along (-x,-y,Z) 1 :=2s-Px+P,-P:] along (-x,y,-Z) h4 中n3 s+px -Py-p: 2 One s and 3 p AO's mix to form a set of along (x,-y,-Z) four hybrid sp3 orbitals. CH,SiH,GeHa,PX,SO,PO d.dsp3(sp3d)hybrides (bipyramidal) n3 1 m= 1 =V2Ip.+d:] 0h2= 12 Vlp:-d:] 1 1 S- 3 6 Px 2H C H H H φh1 φh2 φh4 φh3 z along (-x,-y,z) 2 [ ] 2 1 h px py pz φ = s − − + along (x, y,z) 1 [ ] 2 1 h x y z φ = s + p + p + p along (x,-y,-z) [ ] 2 1 h 4 x y z φ = s + p − p − p along (-x,y,-z) 3 [ ] 2 1 h px py pz φ = s − + − • One s and 3 p AO’s mix to form a set of four hybrid sp3 orbitals. equivalent hybridization α=1/4 c. sp3 hybrides (tetrahedral) − 3− 4 2 4 4 4 4 4 CH , SiH ,GeH , PX , SO , PO P F F F F F h x y h x y h x s p p s p p s p 2 1 6 1 3 1 2 1 6 1 3 1 3 2 3 1 3 2 1 = − − = − + = + φ φ φ φh1 φh2 x y φh3 [ ] 2 1 [ ] 2 1 2 2 5 4 z h z z h z p d p d = − = + φ φ φh4 φh5 z S F F F F d. dsp3 (sp3d) hybrides (bipyramidal)