正在加载图片...
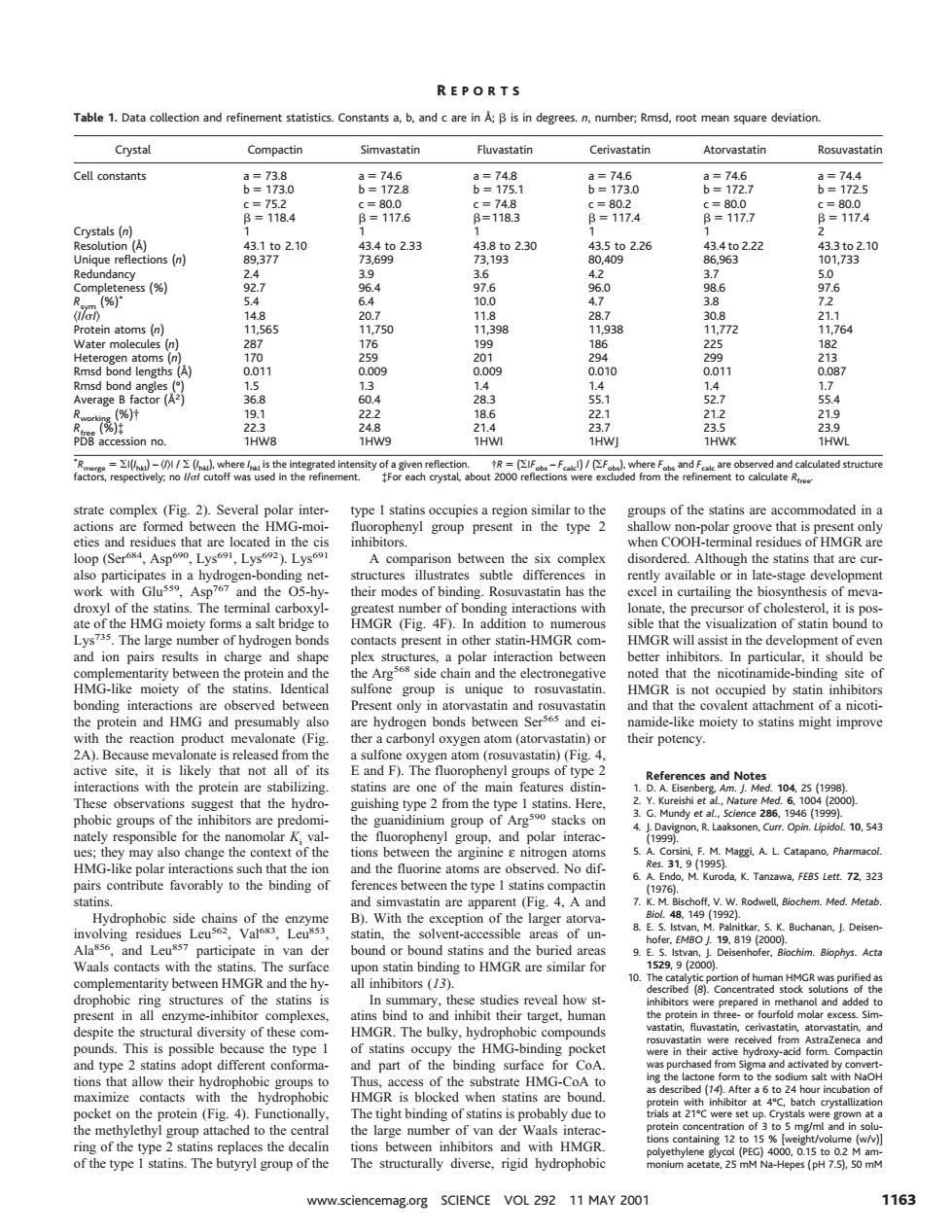
REPORTS Table 1.Data collectio a.b.and c are in:B is in degrees. number:Rmsd.roc deviation Crystal Compactin Simvastatin Fluvastatin Cerivastatin Atorvastatin Rosuvastati Cell constants 8=5 8-1176 6=974 ue re tions (n) ,9230 8050o2.26 964622 s(% 0 01 289 strate complex (Fig. .2).Sev of the statinsre mmodated in and ues th are ated in the cis hen co F HMGR are esna hydrog ond ures illustr available or in late. staee de the -hy ing tatin The the Fig T IMGE nof statin nd ion pairs results in charge dhactionbcn h of the tins. unique upicd by statin tcin and HMG and p ably also and ei mid-like moiety to statins might improve g fo )( nd I obs 309 shing t Jh 10.543 sibl olar inte Pharmaco MG-kpolar nd the fluorin ms are ibut a,K.Tanzawa,FEBS Lett.72.323 4.A Med.e Hydrophobic of the ato and Leu articipate van der fo tarity between HMGR and the hy The bulk ds.Th part of ing surface naximiz ontacts iydopiob a6t02 g12 of th The W.sciencemag.org SCIENCE VOL 292 11 MAY 200 1163strate complex (Fig. 2). Several polar interactions are formed between the HMG-moieties and residues that are located in the cis loop (Ser684, Asp690, Lys691, Lys692). Lys691 also participates in a hydrogen-bonding network with Glu559, Asp767 and the O5-hydroxyl of the statins. The terminal carboxylate of the HMG moiety forms a salt bridge to Lys735. The large number of hydrogen bonds and ion pairs results in charge and shape complementarity between the protein and the HMG-like moiety of the statins. Identical bonding interactions are observed between the protein and HMG and presumably also with the reaction product mevalonate (Fig. 2A). Because mevalonate is released from the active site, it is likely that not all of its interactions with the protein are stabilizing. These observations suggest that the hydrophobic groups of the inhibitors are predominately responsible for the nanomolar Ki values; they may also change the context of the HMG-like polar interactions such that the ion pairs contribute favorably to the binding of statins. Hydrophobic side chains of the enzyme involving residues Leu562, Val683, Leu853, Ala856, and Leu857 participate in van der Waals contacts with the statins. The surface complementarity between HMGR and the hydrophobic ring structures of the statins is present in all enzyme-inhibitor complexes, despite the structural diversity of these compounds. This is possible because the type 1 and type 2 statins adopt different conformations that allow their hydrophobic groups to maximize contacts with the hydrophobic pocket on the protein (Fig. 4). Functionally, the methylethyl group attached to the central ring of the type 2 statins replaces the decalin of the type 1 statins. The butyryl group of the type 1 statins occupies a region similar to the fluorophenyl group present in the type 2 inhibitors. A comparison between the six complex structures illustrates subtle differences in their modes of binding. Rosuvastatin has the greatest number of bonding interactions with HMGR (Fig. 4F). In addition to numerous contacts present in other statin-HMGR complex structures, a polar interaction between the Arg568 side chain and the electronegative sulfone group is unique to rosuvastatin. Present only in atorvastatin and rosuvastatin are hydrogen bonds between Ser565 and either a carbonyl oxygen atom (atorvastatin) or a sulfone oxygen atom (rosuvastatin) (Fig. 4, E and F). The fluorophenyl groups of type 2 statins are one of the main features distinguishing type 2 from the type 1 statins. Here, the guanidinium group of Arg590 stacks on the fluorophenyl group, and polar interactions between the arginine ε nitrogen atoms and the fluorine atoms are observed. No differences between the type 1 statins compactin and simvastatin are apparent (Fig. 4, A and B). With the exception of the larger atorvastatin, the solvent-accessible areas of unbound or bound statins and the buried areas upon statin binding to HMGR are similar for all inhibitors (13). In summary, these studies reveal how statins bind to and inhibit their target, human HMGR. The bulky, hydrophobic compounds of statins occupy the HMG-binding pocket and part of the binding surface for CoA. Thus, access of the substrate HMG-CoA to HMGR is blocked when statins are bound. The tight binding of statins is probably due to the large number of van der Waals interactions between inhibitors and with HMGR. The structurally diverse, rigid hydrophobic groups of the statins are accommodated in a shallow non-polar groove that is present only when COOH-terminal residues of HMGR are disordered. Although the statins that are currently available or in late-stage development excel in curtailing the biosynthesis of mevalonate, the precursor of cholesterol, it is possible that the visualization of statin bound to HMGR will assist in the development of even better inhibitors. In particular, it should be noted that the nicotinamide-binding site of HMGR is not occupied by statin inhibitors and that the covalent attachment of a nicotinamide-like moiety to statins might improve their potency. References and Notes 1. D. A. Eisenberg, Am. J. Med. 104, 2S (1998). 2. Y. Kureishi et al., Nature Med. 6, 1004 (2000). 3. G. Mundy et al., Science 286, 1946 (1999). 4. J. Davignon, R. Laaksonen, Curr. Opin. Lipidol. 10, 543 (1999). 5. A. Corsini, F. M. Maggi, A. L. Catapano, Pharmacol. Res. 31, 9 (1995). 6. A. Endo, M. Kuroda, K. Tanzawa, FEBS Lett. 72, 323 (1976). 7. K. M. Bischoff, V. W. Rodwell, Biochem. Med. Metab. Biol. 48, 149 (1992). 8. E. S. Istvan, M. Palnitkar, S. K. Buchanan, J. Deisenhofer, EMBO J. 19, 819 (2000). 9. E. S. Istvan, J. Deisenhofer, Biochim. Biophys. Acta 1529, 9 (2000). 10. The catalytic portion of human HMGR was purified as described (8). Concentrated stock solutions of the inhibitors were prepared in methanol and added to the protein in three- or fourfold molar excess. Simvastatin, fluvastatin, cerivastatin, atorvastatin, and rosuvastatin were received from AstraZeneca and were in their active hydroxy-acid form. Compactin was purchased from Sigma and activated by converting the lactone form to the sodium salt with NaOH as described (14). After a 6 to 24 hour incubation of protein with inhibitor at 4°C, batch crystallization trials at 21°C were set up. Crystals were grown at a protein concentration of 3 to 5 mg/ml and in solutions containing 12 to 15 % [weight/volume (w/v)] polyethylene glycol (PEG) 4000, 0.15 to 0.2 M ammonium acetate, 25 mM Na-Hepes (pH 7.5), 50 mM Table 1. Data collection and refinement statistics. Constants a, b, and c are in Å; b is in degrees. n, number; Rmsd, root mean square deviation. Crystal Compactin Simvastatin Fluvastatin Cerivastatin Atorvastatin Rosuvastatin Cell constants a 5 73.8 b 5 173.0 c 5 75.2 b 5 118.4 a 5 74.6 b 5 172.8 c 5 80.0 b 5 117.6 a 5 74.8 b 5 175.1 c 5 74.8 b5118.3 a 5 74.6 b 5 173.0 c 5 80.2 b 5 117.4 a 5 74.6 b 5 172.7 c 5 80.0 b 5 117.7 a 5 74.4 b 5 172.5 c 5 80.0 b 5 117.4 Crystals (n) 111112 Resolution (Å) 43.1 to 2.10 43.4 to 2.33 43.8 to 2.30 43.5 to 2.26 43.4 to 2.22 43.3 to 2.10 Unique reflections (n) 89,377 73,699 73,193 80,409 86,963 101,733 Redundancy 2.4 3.9 3.6 4.2 3.7 5.0 Completeness (%) 92.7 96.4 97.6 96.0 98.6 97.6 Rsym (%)* 5.4 6.4 10.0 4.7 3.8 7.2 ^I/sI& 14.8 20.7 11.8 28.7 30.8 21.1 Protein atoms (n) 11,565 11,750 11,398 11,938 11,772 11,764 Water molecules (n) 287 176 199 186 225 182 Heterogen atoms (n) 170 259 201 294 299 213 Rmsd bond lengths (Å) 0.011 0.009 0.009 0.010 0.011 0.087 Rmsd bond angles (°) 1.5 1.3 1.4 1.4 1.4 1.7 Average B factor (Å2) 36.8 60.4 28.3 55.1 52.7 55.4 Rworking (%)† 19.1 22.2 18.6 22.1 21.2 21.9 Rfree (%)‡ 22.3 24.8 21.4 23.7 23.5 23.9 PDB accession no. 1HW8 1HW9 1HWI 1HWJ 1HWK 1HWL * Rmerge 5 S?(I hkl) – ^I&? / S (I hkl), where I hkl is the integrated intensity of a given reflection. †R 5 (S?Fobs – Fcalc?)/(SFobs), where Fobs and Fcalc are observed and calculated structure factors, respectively; no I/sI cutoff was used in the refinement. ‡For each crystal, about 2000 reflections were excluded from the refinement to calculate Rfree. R EPORTS www.sciencemag.org SCIENCE VOL 292 11 MAY 2001 1163