正在加载图片...
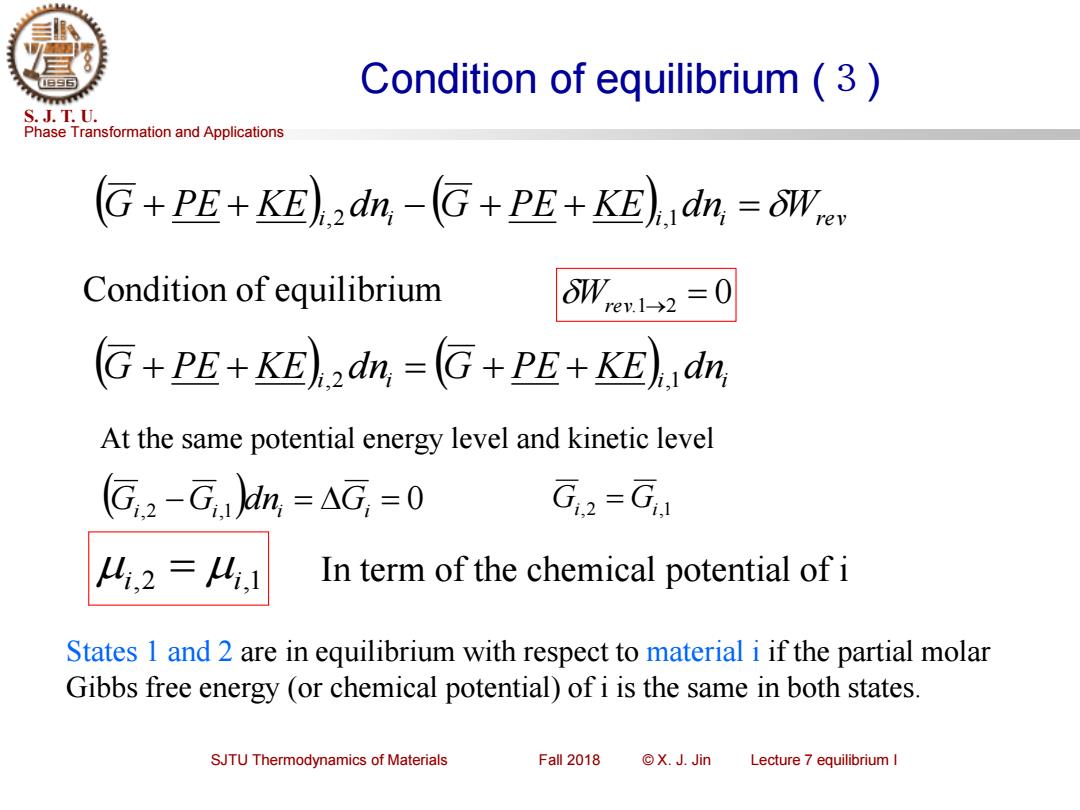
Condition of equilibrium(3) S.J.T.U. Phase Transformation and Applications G+PE+KE)dn,-G+PE+KE)dn,=OW Condition of equilibrium W,e1-→2=0 G+PE+KE)dn,=G+PE+KE)dn, At the same potential energy level and kinetic level (G,2-G,)dn,=AG,=0 G,2-G 4,2=4,1 In term of the chemical potential of i States 1 and 2 are in equilibrium with respect to material i if the partial molar Gibbs free energy (or chemical potential)of i is the same in both states. SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium IPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Condition of equilibrium (3) ( ) ( ) G + PE + KE i,2 d ni − G + PE + KE i,1 d ni = Wrev Condition of equilibrium Wrev.1→2 = 0 ( ) ( ) G + PE + KE i,2 d ni = G + PE + KE i,1 d ni (Gi,2 −Gi,1 )dni = Gi = 0 Gi,2 = Gi,1 At the same potential energy level and kinetic level i,2 = i,1 In term of the chemical potential of i States 1 and 2 are in equilibrium with respect to material i if the partial molar Gibbs free energy (or chemical potential) of i is the same in both states