正在加载图片...
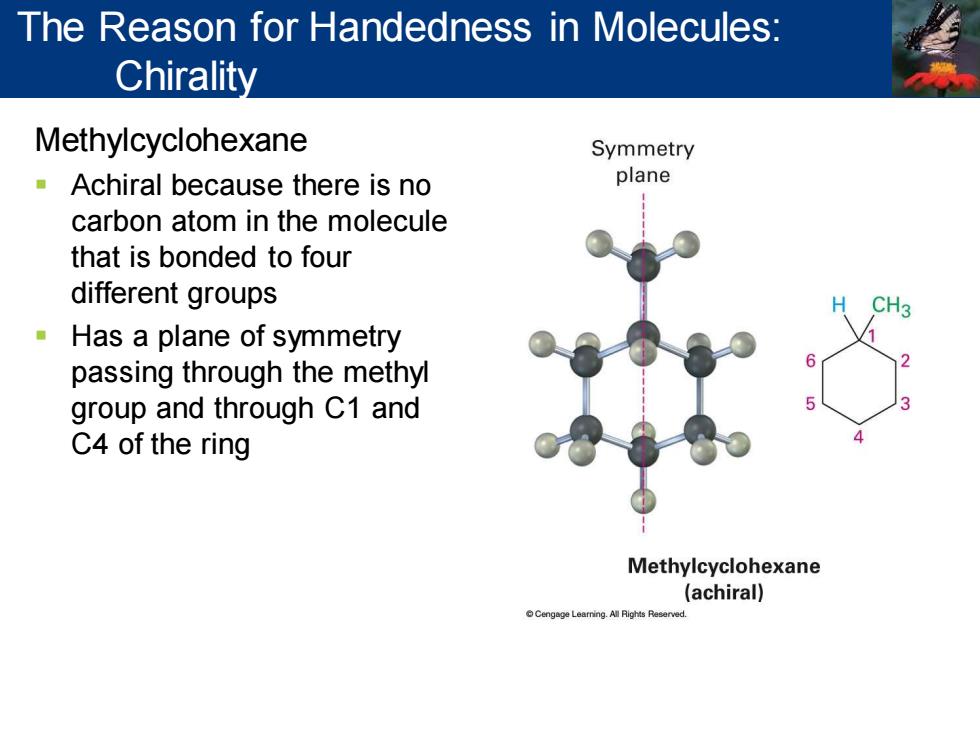
The Reason for Handedness in Molecules: Chirality Methylcyclohexane Symmetry Achiral because there is no plane carbon atom in the molecule that is bonded to four different groups Has a plane of symmetry passing through the methyl group and through C1 and C4 of the ring Methylcyclohexane (achiral) Cengege Learning.All Rights Reserved Methylcyclohexane ▪ Achiral because there is no carbon atom in the molecule that is bonded to four different groups ▪ Has a plane of symmetry passing through the methyl group and through C1 and C4 of the ring The Reason for Handedness in Molecules: Chirality