正在加载图片...
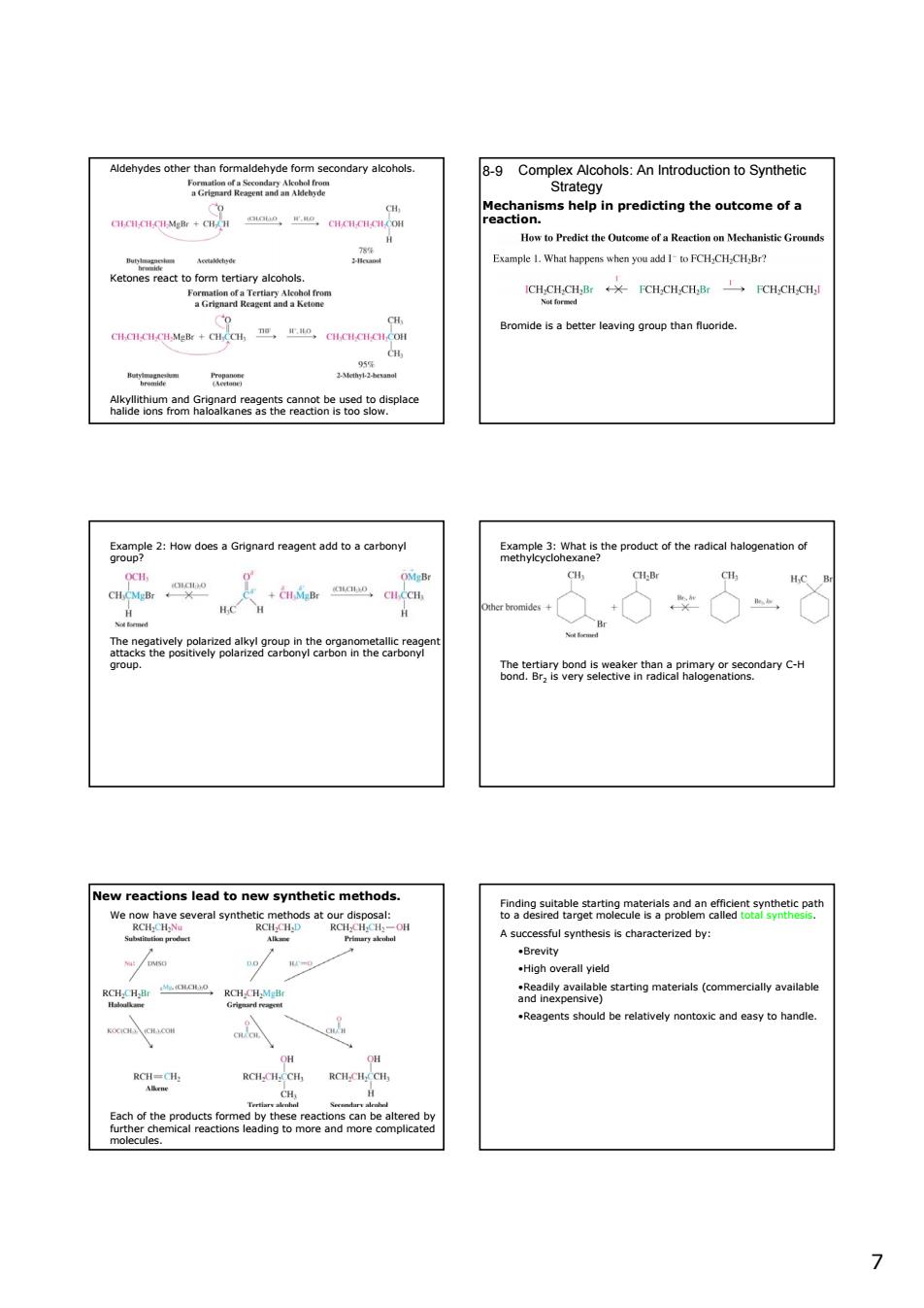
Aldehydesth tha form dehyde fo condary alcohols 8-9 Complex Alcohols:An Introduction to Synthetic Example 1.What happens htFCHCHCHBr? Ketonesreattofomtetiayakohck CTanm CHGH¥CH,CHCHB FCH.CH.CE Bromide is a better leaving group than fluoride w eoS8Sa5Bepoe 50pge2zHowdosaGngnadreaoent8datoacartoa metheucto theradica CH:Br Ge w oeaeEg8eecaerntad8aPn8egosloeamdayc-H reactions lead to new synthetic methods ssful synthesis is characterized by: RCI-C 7 7 Aldehydes other than formaldehyde form secondary alcohols. Ketones react to form tertiary alcohols. Alkyllithium and Grignard reagents cannot be used to displace halide ions from haloalkanes as the reaction is too slow. Complex Alcohols: An Introduction to Synthetic Strategy 8-9 Mechanisms help in predicting the outcome of a reaction. Bromide is a better leaving group than fluoride. The negatively polarized alkyl group in the organometallic reagent attacks the positively polarized carbonyl carbon in the carbonyl group. Example 2: How does a Grignard reagent add to a carbonyl group? The tertiary bond is weaker than a primary or secondary C-H bond. Br2 is very selective in radical halogenations. Example 3: What is the product of the radical halogenation of methylcyclohexane? New reactions lead to new synthetic methods. We now have several synthetic methods at our disposal: Each of the products formed by these reactions can be altered by further chemical reactions leading to more and more complicated molecules. Finding suitable starting materials and an efficient synthetic path to a desired target molecule is a problem called total synthesis. A successful synthesis is characterized by: •Brevity •High overall yield •Readily available starting materials (commercially available and inexpensive) •Reagents should be relatively nontoxic and easy to handle