正在加载图片...
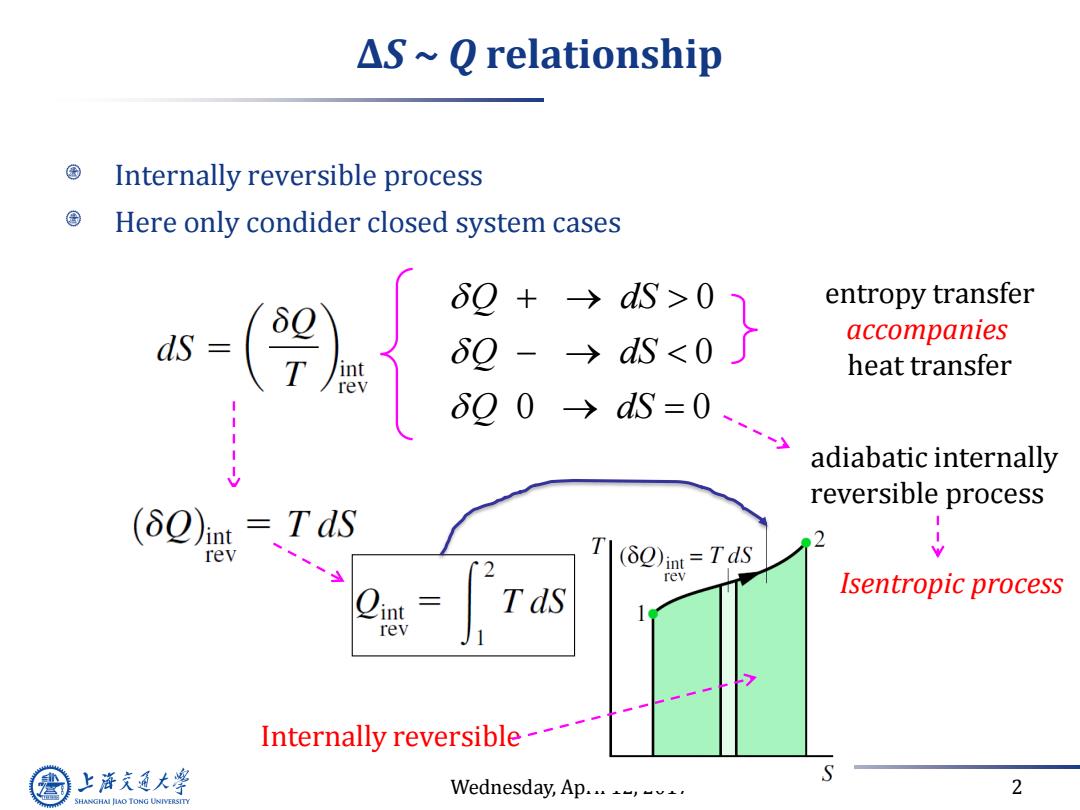
△S~Q relationship Internally reversible process Here only condider closed system cases 6Q+→dS>0 entropy transfer 6g-→dS<0 accompanies heat transfer 00→S=0y adiabatic internally reversible process (δQ)mt=TdS rev 2 T(2)in=Tds rev Tds Isentropic process rev Internally reversible--- 上游充通大 Wednesday,Ap...-,. 2 SHANGHAI JIAO TONG UNIVERSITYWednesday, April 12, 2017 2 ∆S ~ Q relationship Internally reversible process Here only condider closed system cases 0 0 0 0 Q dS Q dS Q dS entropy transfer accompanies heat transfer adiabatic internally reversible process Isentropic process Internally reversible