正在加载图片...
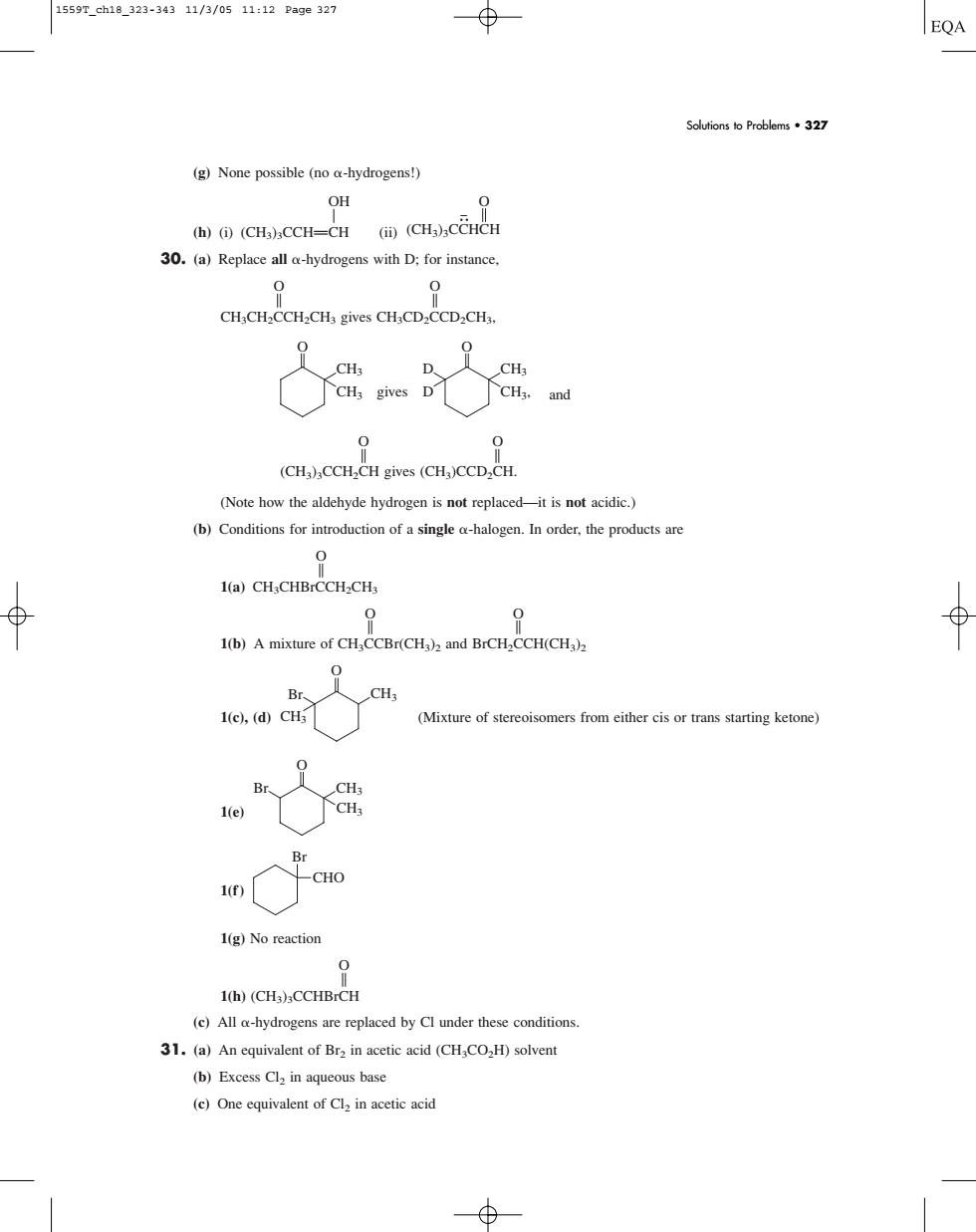
1559r.ch10323-34311/3/0s11:12Page327 EQA Solutions to Problems37 (g)None possible(noa-hydrogens!) OH (h)()(CH)CCH-CH ((CH)CCHCH 30.(a)Replace all a-hydrogens with D:for instance. 0 CH.CH.CCH-CH,gives CH.CD.CCD.CH 品品 D 0 (CHa)CCH.CH gives (CHJCCD,CH. (Note how the aldehyde hydrogen is not replaced-it is not acidic.) (b)Conditions for introduction of a single a-halogen.In order,the products are 1(a)CH:CHBrCCH-CH, 1(b)A mixture of CH,CCBr(CH)and BrCH,CCH(CH) 0 (Mixture of stereoisomers from either cis or trans starting ketone) B Br 1(g)No reaction 1(h)(CH.CCHBrCH (c)All o-hydrogens are replaced by Cl under these conditions 31.(a)An equivalent of Bra in acetic acid (CHCO,H)solvent (b)Excess Cl2 in aqueous base (e)One equivalent of Clin acetic acid(g) None possible (no -hydrogens!) OH A (h) (i) (CH3)3CCHPCH (ii) 30. (a) Replace all -hydrogens with D; for instance, O O B B CH3CH2CCH2CH3 gives CH3CD2CCD2CH3, and O O B B (CH3)3CCH2CH gives (CH3)CCD2CH. (Note how the aldehyde hydrogen is not replaced—it is not acidic.) (b) Conditions for introduction of a single -halogen. In order, the products are O B 1(a) CH3CHBrCCH2CH3 O O B B 1(b) A mixture of CH3CCBr(CH3)2 and BrCH2CCH(CH3)2 1(c), (d) (Mixture of stereoisomers from either cis or trans starting ketone) 1(e) 1(f) 1(g) No reaction O B 1(h) (CH3)3CCHBrCH (c) All -hydrogens are replaced by Cl under these conditions. 31. (a) An equivalent of Br2 in acetic acid (CH3CO2H) solvent (b) Excess Cl2 in aqueous base (c) One equivalent of Cl2 in acetic acid CHO Br CH3 O Br CH3 CH3 CH3 O Br O CH3 CH3 gives CH3 CH3, O D D O (CH3)3CCHCH Solutions to Problems • 327 1559T_ch18_323-343 11/3/05 11:12 Page 327