正在加载图片...
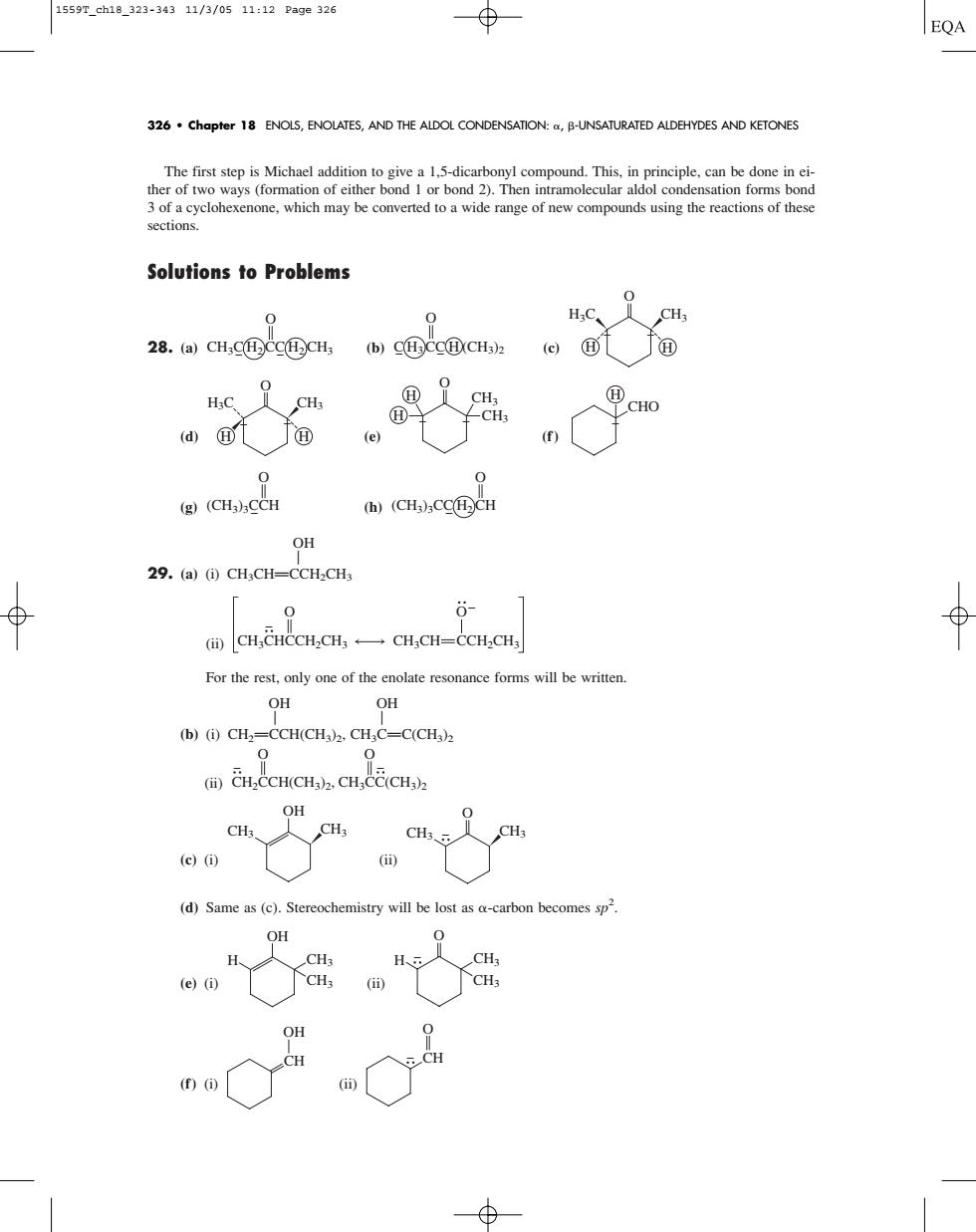
1559T_ch18_323-34311/3/0511:12Page326 ⊕ EQA 326 Chapter 18 ENOLS,ENOLATES,AND THE ALDOL CONDENSATION:B-UNSATURATED ALDEHYDES AND KETONES The first step is michael addition to give a 15-dicarbonyl comr 3 of a cyclohexenone.which may be converted toa wide range of new compounds using the reactions of thes Solutions to Problems 0 HC、 28.(a)CHC①Sc①CH bc①cc①cHhd@j@ CHO (e) ((CH3)CCH (h)(CH3).CCCH OH 29.(a)(i)CHCH=CCH-CH, 0 0- mCH,CHCCH,CH,←一CH,CH=CCH,CH For the rest,only one of the enolate resonance forms will be writter OH OH (b)(i)CH2-CCH(CH3)2.CH;C-C(CHs)z 0 CH.CCH(CHCH.CE(CH CH (d)Same as(c).Stereochemistry will be lost as a-carbon becomes sp OH e)(i) Gi) The first step is Michael addition to give a 1,5-dicarbonyl compound. This, in principle, can be done in either of two ways (formation of either bond 1 or bond 2). Then intramolecular aldol condensation forms bond 3 of a cyclohexenone, which may be converted to a wide range of new compounds using the reactions of these sections. Solutions to Problems 28. (a) (b) (c) (d) (e) (f) (g) (h) OH A 29. (a) (i) CH3CHPCCH2CH3 (ii) For the rest, only one of the enolate resonance forms will be written. OH OH A A (b) (i) CH2PCCH(CH3)2, CH3CPC(CH3)2 (ii) (c) (i) (ii) (d) Same as (c). Stereochemistry will be lost as -carbon becomes sp2 . (e) (i) (ii) (f) (i) (ii) O CH OH CH H CH3 CH3 OH O H CH3 CH3 CH3 CH3 O OH CH3 CH3 CH2CCH(CH3)2, CH3CC(CH3)2 O O CH3CHCCH2CH3 CH3CH CCH2CH3 O O (CH3)3CC H2CH O (CH3)3CCH O CHO H O H CH3 CH3 H H3C CH3 O H H H3C CH3 O H H C H3CC H (CH3)2 O CH3C H2CC H2CH3 O 326 • Chapter 18 ENOLS, ENOLATES, AND THE ALDOL CONDENSATION: , -UNSATURATED ALDEHYDES AND KETONES 1559T_ch18_323-343 11/3/05 11:12 Page 326�