正在加载图片...
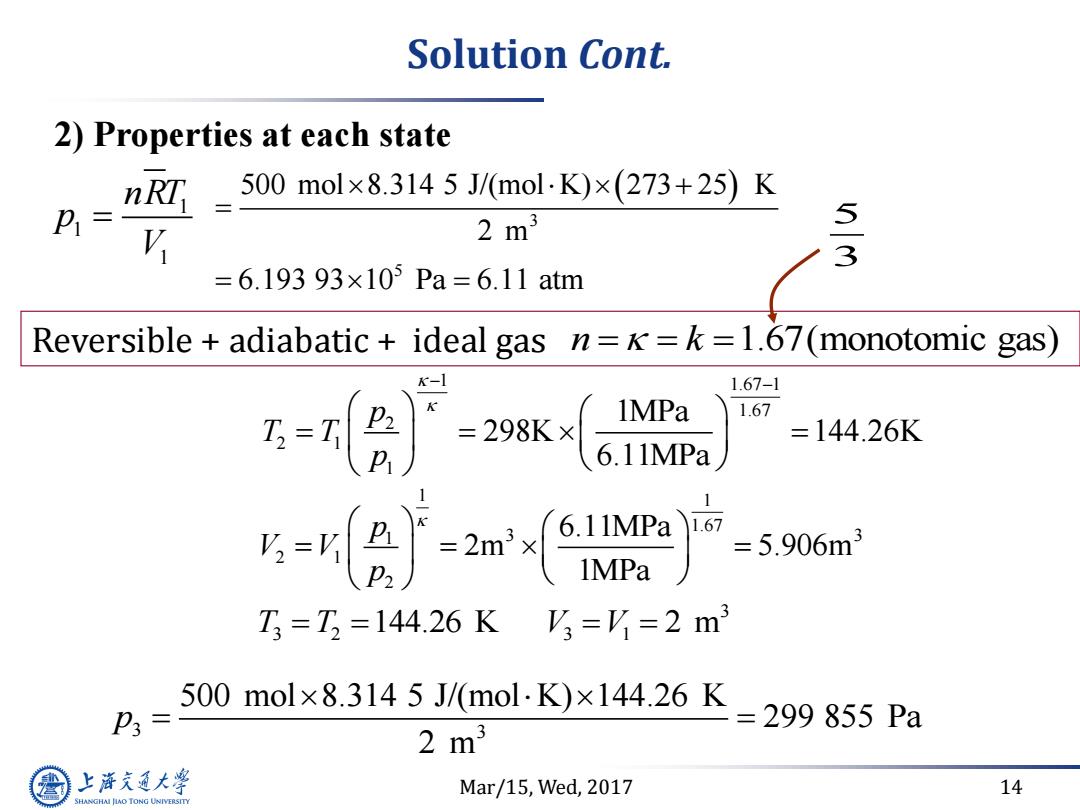
Solution Cont. 2)Properties at each state 500mol×8.3145J/(mol.K)×273+25)K P1= V 2m3 5 3 =6.19393×105Pa=6.11atm Reversible adiabatic ideal gas n==k=1.67(monotomic gas) K-1 1.67-1 1MPa 1.67 ,= =144.26K =-2)产 1.67 5.906m3 T=T,=144.26KV3=Y=2m3 500mol×8.3145J/(mol.K)×144.26K P3= 2m3 =299855Pa 上游充通大 Mar/15,Wed,2017 14 SHANGHAI JLAO TONG UNIVERSITYMar/15, Wed, 2017 14 1 1 1 nRT p V 3 3 2 3 1 T T V V 144.26 K 2 m 1 1.67 1 1.67 2 2 1 1 1MPa 298K 144.26K 6.11MPa p T T p 1 1 1.67 1 3 3 2 1 2 6.11MPa 2m 5.906m 1MPa p V V p 3 5 500 mol 8.314 5 J/(mol K) 273 25 K 2 m 6.193 93 10 Pa 6.11 atm 3 3 500 mol 8.314 5 J/(mol K) 144.26 K 299 855 Pa 2 m p Reversible + adiabatic + ideal gas n k 1.67(monotomic gas) 2) Properties at each state Solution Cont. 5 3