正在加载图片...
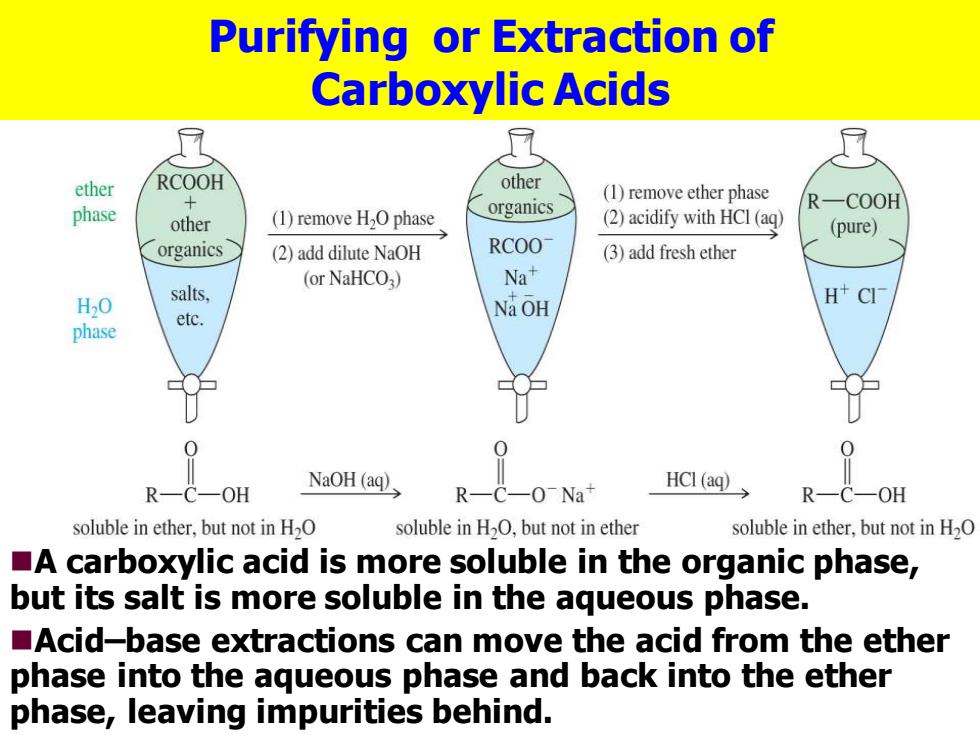
Purifying or Extraction of Carboxylic Acids ether RCOOH other (1)remove ether phase phase organics R-COOH other (1)remove H2O phase (2)acidify with HCI(aq) (pure) organics (2)add dilute NaOH RCOO (3)add fresh ether (or NaHCO3) Na+ H2O salts, H CI etc. Na OH phase 0 0 R-C-OH NaOH (aq) R-C-0-Na+ HCl (aq) R—C-OH soluble in ether,but not in H2O soluble in H2O,but not in ether soluble in ether,but not in H2O A carboxylic acid is more soluble in the organic phase, but its salt is more soluble in the aqueous phase. Acid-base extractions can move the acid from the ether phase into the aqueous phase and back into the ether phase,leaving impurities behind. Purifying or Extraction of Carboxylic Acids ◼A carboxylic acid is more soluble in the organic phase, but its salt is more soluble in the aqueous phase. ◼Acid–base extractions can move the acid from the ether phase into the aqueous phase and back into the ether phase, leaving impurities behind