正在加载图片...
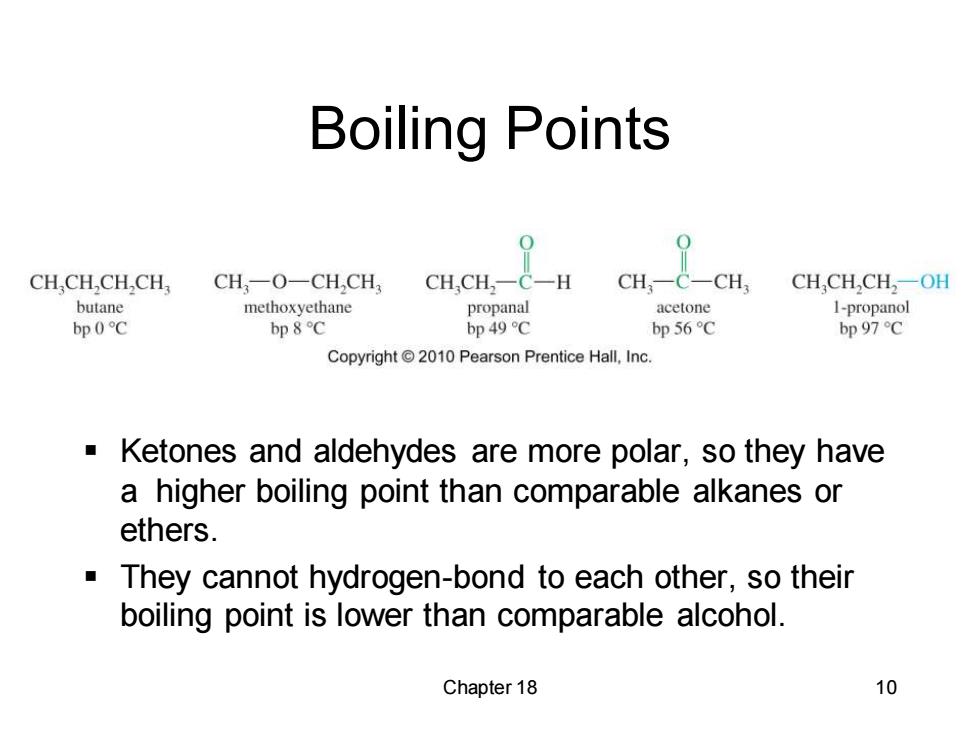
Boiling Points CH.CH,CH,CH, CH一O一CHCH CH,CH2一C一H CH,- C一CH CH,CH,CH2一OH butane methoxyethane propanal acetone I-propanol bp0℃ bp 8C bp 49 C bp 56C bp 97C Copyright2010 Pearson Prentice Hall,Inc. Ketones and aldehydes are more polar,so they have a higher boiling point than comparable alkanes or ethers. They cannot hydrogen-bond to each other,so their boiling point is lower than comparable alcohol. Chapter 18 10Chapter 18 10 Boiling Points ▪ Ketones and aldehydes are more polar, so they have a higher boiling point than comparable alkanes or ethers. ▪ They cannot hydrogen-bond to each other, so their boiling point is lower than comparable alcohol