正在加载图片...
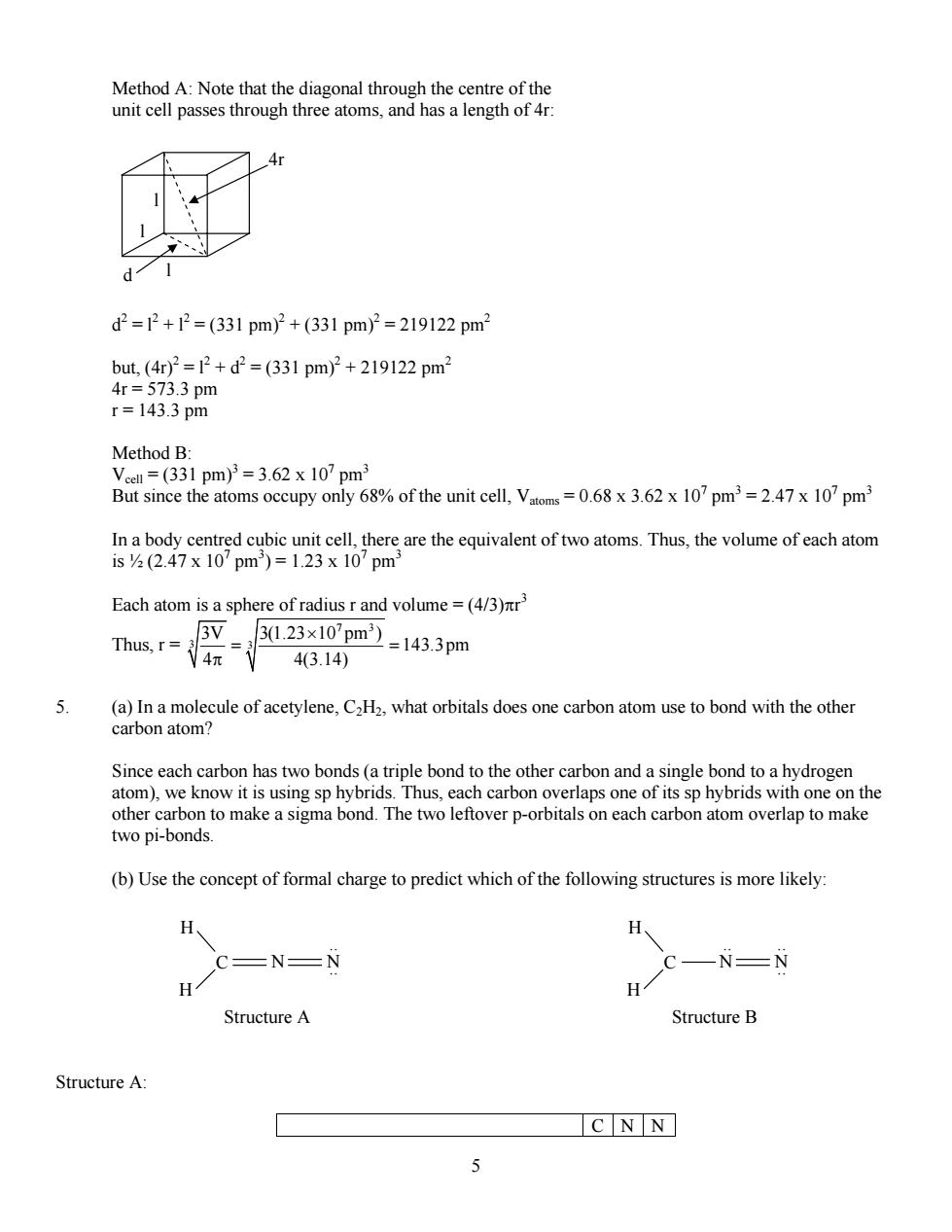
Method A:Note that the diagonal through the centre of th unit cell passes through three atoms,and has a length of 4 d2=2+2=(331pm)2+(331pm2=219122pm2 but(4r)2=2+d=(331pm)2+219122pm3 4r=573.3pm r=143.3pm Method B: 33 pm)=3.62 x107pm unit cell V10'pm71m In a body centred cubic unit cell,there are the equivalent of two atoms.Thus,the volume of each atom 1sy2(2.47X10Dm)=1.23X10'Dm Each atom is a sphere of radius r and volume=(4/3)nr' Thus,r= 3V 玩 3023×10pm-143.3pm 43.14) (a)In a molecule of acetylene,C2H2,what orbitals does one carbon atom use to bond with the other carbon atom? Since each carbon has two bonds(a triple bond to the other carbon and a single bond to a hydrogen atom),we know it is using sp hybrids.Thus,each carbon overlaps one of its sp hybrids with one on the other carbon to make a sigma bond.The two leftover p-orbitals on each carbon atom overlap to make two pi-bonds. (b)Use the concept of formal charge to predict which of the following structures is more likely: C=N=N -N-N H H StructureA Structure B Structure A C NN 55 Method A: Note that the diagonal through the centre of the unit cell passes through three atoms, and has a length of 4r: l l l d 4r d2 = l2 + l2 = (331 pm)2 + (331 pm)2 = 219122 pm2 but, (4r)2 = l2 + d2 = (331 pm)2 + 219122 pm2 4r = 573.3 pm r = 143.3 pm Method B: Vcell = (331 pm)3 = 3.62 x 107 pm3 But since the atoms occupy only 68% of the unit cell, Vatoms = 0.68 x 3.62 x 107 pm3 = 2.47 x 107 pm3 In a body centred cubic unit cell, there are the equivalent of two atoms. Thus, the volume of each atom is ½ (2.47 x 107 pm3 ) = 1.23 x 107 pm3 Each atom is a sphere of radius r and volume = (4/3)πr 3 Thus, r = 7 3 3 3 3V 3(1.23 10 pm ) 143.3pm 4 4(3.14) × = = π 5. (a) In a molecule of acetylene, C2H2, what orbitals does one carbon atom use to bond with the other carbon atom? Since each carbon has two bonds (a triple bond to the other carbon and a single bond to a hydrogen atom), we know it is using sp hybrids. Thus, each carbon overlaps one of its sp hybrids with one on the other carbon to make a sigma bond. The two leftover p-orbitals on each carbon atom overlap to make two pi-bonds. (b) Use the concept of formal charge to predict which of the following structures is more likely: C H H N N .. .. C H H N N.. .. .. Structure A Structure B Structure A: C N N