正在加载图片...
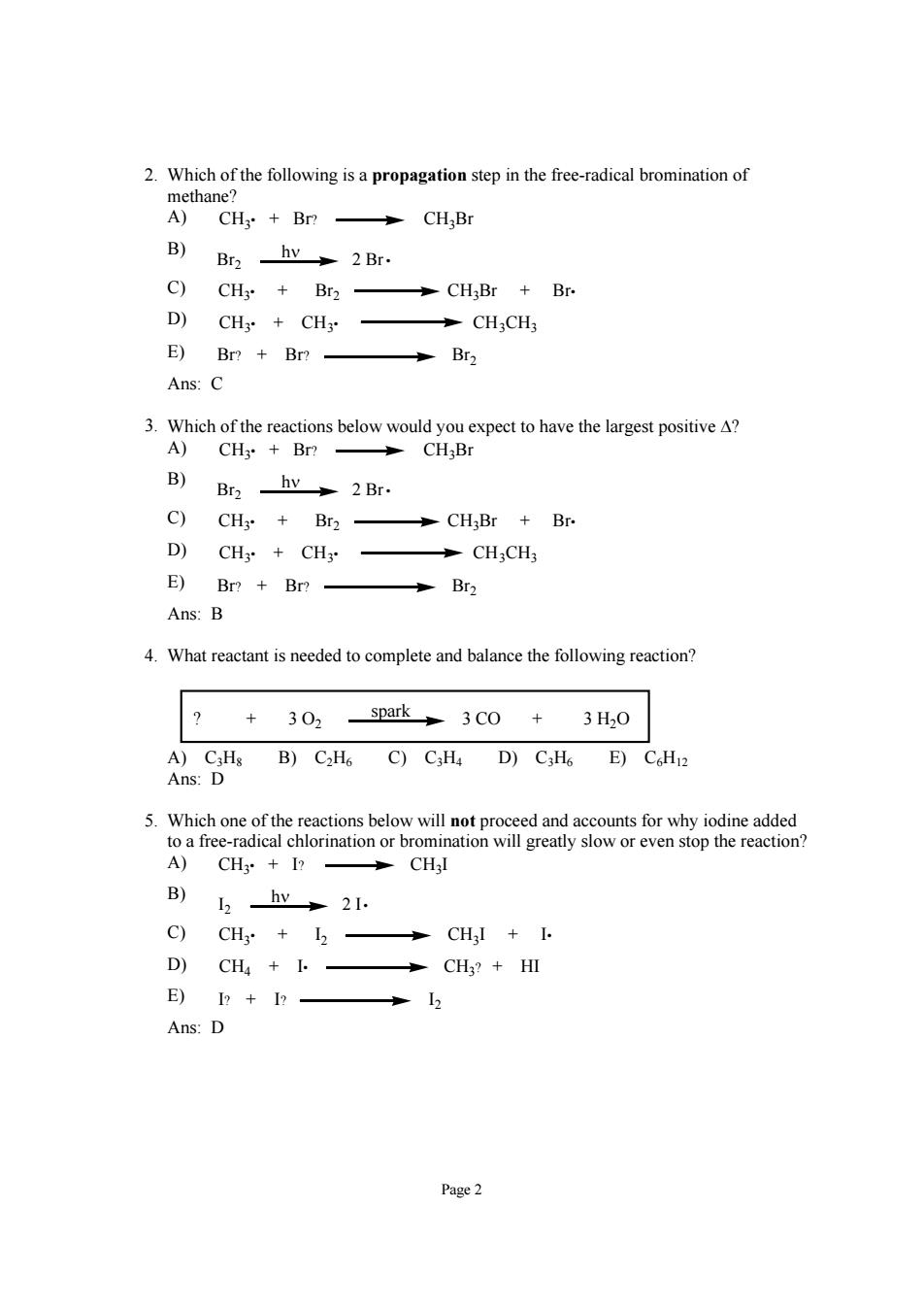
2.Which of the following is a propagation step in the free-radical bromination of B) Bra hy 2Br. C)CH3+Br2- CH;Br Br. D)CHy+CH: CH3CH3 E)Br?+Br? Br2 Ans:C 3.Which of the reactions below would you expect to have the largest positive A? A) CH:Br B) Br2 hy 2 Br. c) CH3 Br2- CH;Br Br. D) CH3CH3 →CH,CH3 E)Br?+Br? Br2 Ans:B 4.What reactant is needed to complete and balance the following reaction? +302spark 3CO +3H0 A)C3Hs B)C2H6 C)C:Ha D)C:H6 E)CH2 Ans:D 5hnenscsbdwnnomgeyawcomtpgti。 A)CH3 I?CHjl B) 2hv+2. C)CH+I- →CH,I+I D)CH+一 →CH?+HⅢ E)I?+I?- 12 Ans:D Page2 Page 2 2. Which of the following is a propagation step in the free-radical bromination of methane? A) CH3• + Br? CH3Br B) Br2 2 Br • hν C) CH3• + Br2 CH3Br + Br• D) CH3• + CH3• CH3CH3 E) Br? + Br? Br2 Ans: C 3. Which of the reactions below would you expect to have the largest positive Δ? A) CH3• + Br? CH3Br B) Br2 2 Br • hν C) CH3• + Br2 CH3Br + Br• D) CH3• + CH3• CH3CH3 E) Br? + Br? Br2 Ans: B 4. What reactant is needed to complete and balance the following reaction? ? + 3 O2 3 CO + 3 H2O spark A) C3H8 B) C2H6 C) C3H4 D) C3H6 E) C6H12 Ans: D 5. Which one of the reactions below will not proceed and accounts for why iodine added to a free-radical chlorination or bromination will greatly slow or even stop the reaction? A) CH3• + I? CH3I B) hν I2 2 I• C) CH3 CH I + I• 3• + I2 D) CH4 + I• CH3? + HI E) I? + I? I2 Ans: D