正在加载图片...
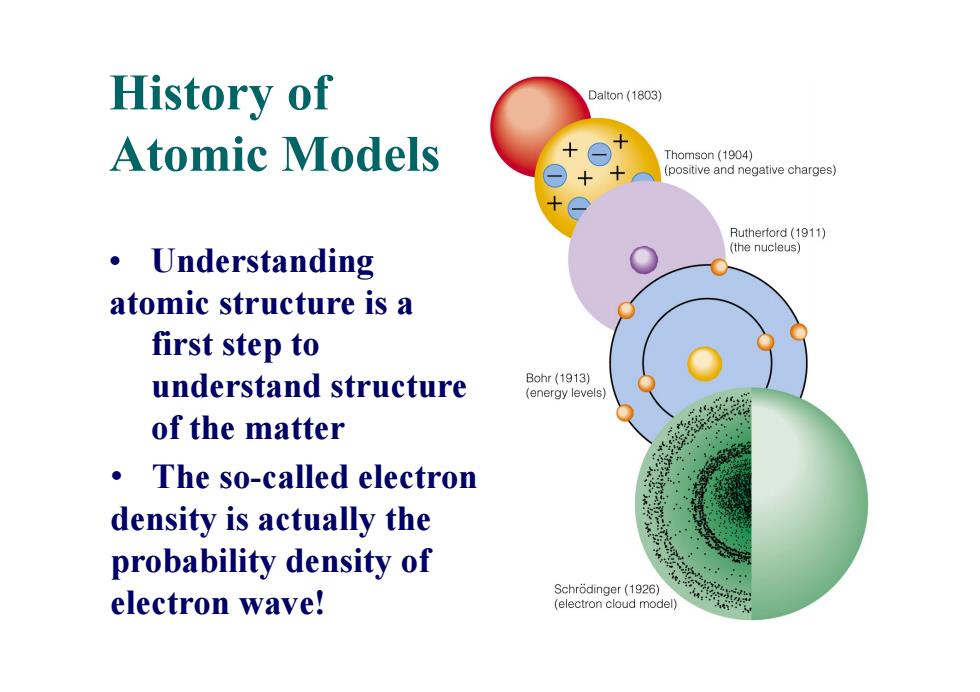
History of Dalton (1803) Atomic Models Thomson(1904) (positive and negative charges) Rutherford(1911) Understanding (the nucleus) 。 atomic structure is a first step to understand structure Bohr(1913) (energy levels) of the matter The so-called electron density is actually the probability density of Schrodinger(1926) electron wave! (electron cloud model)History of Atomic Models • Understanding atomic structure is a first step to understand structure of the matter • The so-called electron density is actually the probability density of electron wave!