正在加载图片...
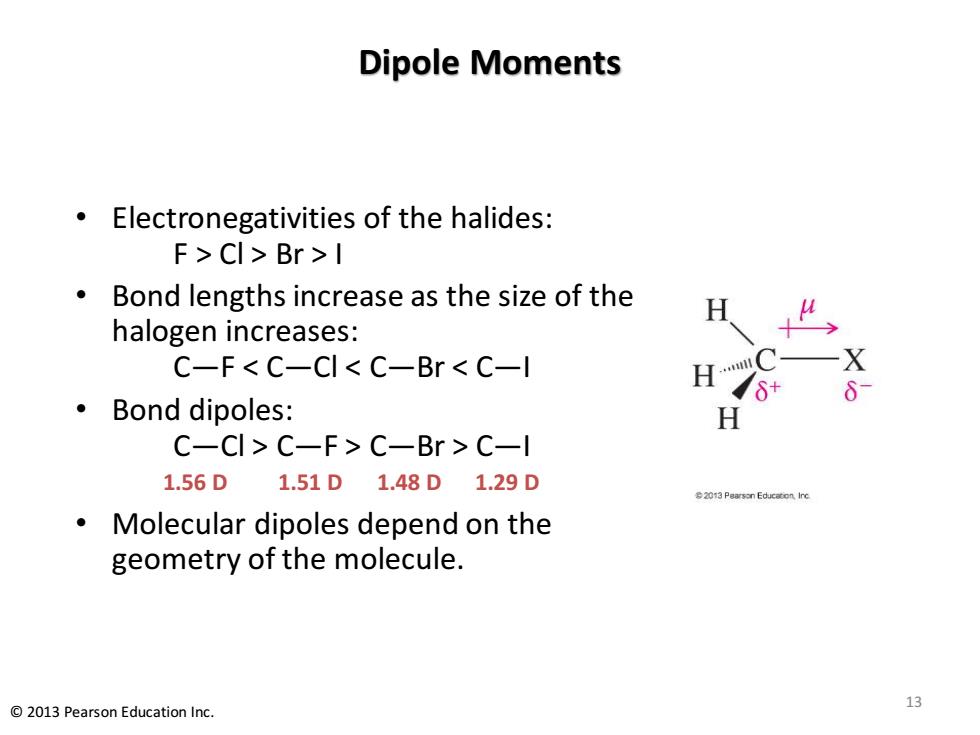
Dipole Moments Electronegativities of the halides: F>CI>Br>I Bond lengths increase as the size of the H halogen increases: C-F<C-CI<C-Br C-l X ·Bond dipoles: H C-CI>C-F>C-Br C-l 1.56D1.51D1.48D1.29D 2013 Pearson Educacon Ine Molecular dipoles depend on the geometry of the molecule. 13 2013 Pearson Education Inc. Dipole Moments • Electronegativities of the halides: F > Cl > Br > I • Bond lengths increase as the size of the halogen increases: C—F < C—Cl < C—Br < C—I • Bond dipoles: C—Cl > C—F > C—Br > C—I 1.56 D 1.51 D 1.48 D 1.29 D • Molecular dipoles depend on the geometry of the molecule. 13 © 2013 Pearson Education Inc