正在加载图片...
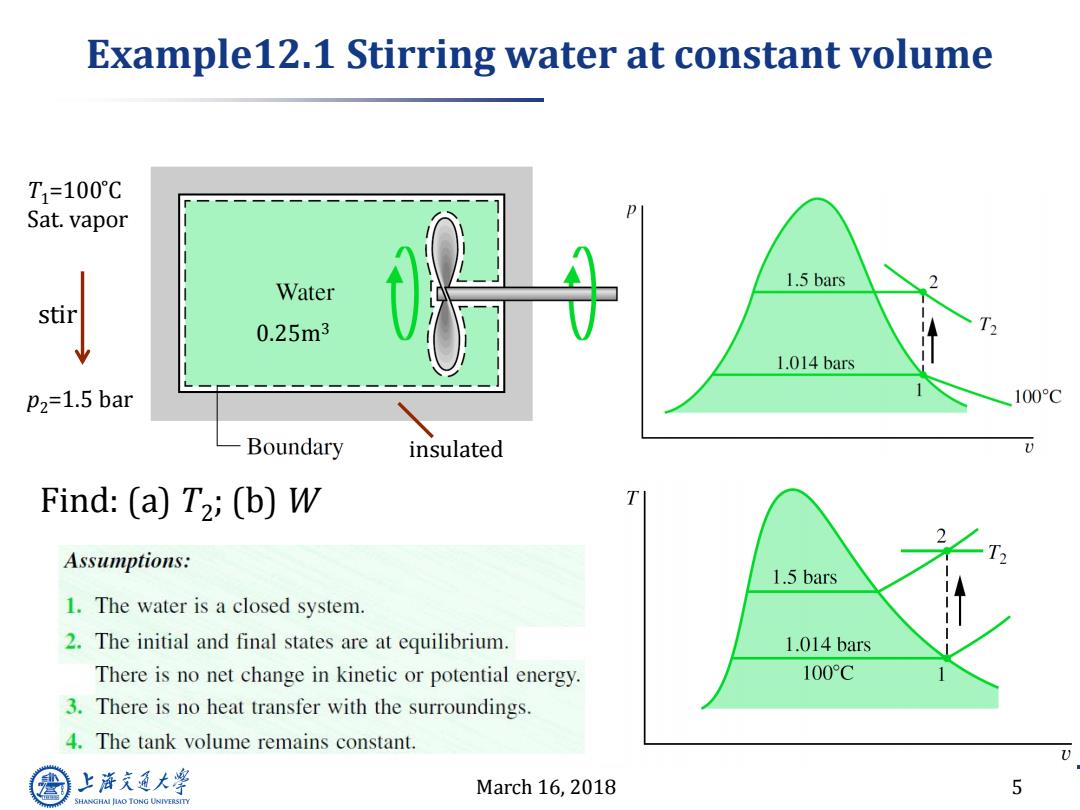
Example12.1 Stirring water at constant volume T1=100℃ Sat.vapor Water 1.5 bars stir 0.25m3 1.014 bars P2=1.5 bar 100C Boundary insulated Find:(a)T2;(b)W Assumptions: 1.5 bars 1.The water is a closed system. 2.The initial and final states are at equilibrium. 1.014 bars There is no net change in kinetic or potential energy. 100°C 3.There is no heat transfer with the surroundings. 4.The tank volume remains constant. 上游充通大 March 16,2018 5 SHANGHAI JLAO TONG UNIVERSITYMarch 16, 2018 5 Example12.1 Stirring water at constant volume insulated 0.25m3 p2=1.5 bar T1=100 ̊C Sat. vapor stir Find: (a) T2 ; (b) W