正在加载图片...
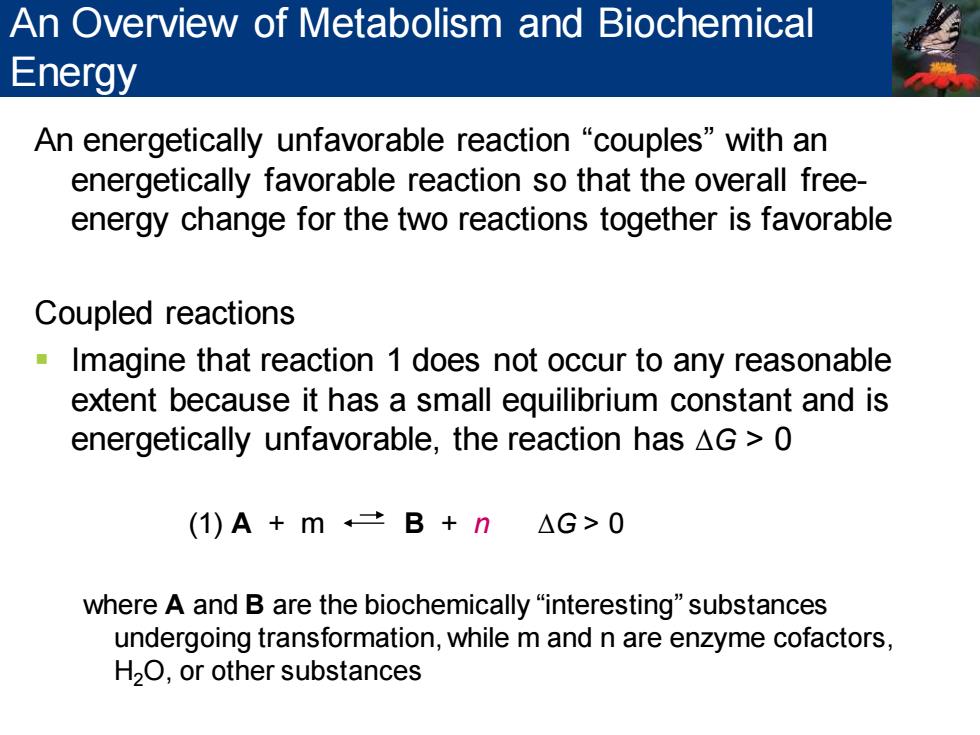
An Overview of Metabolism and Biochemical Energy An energetically unfavorable reaction "couples"with an energetically favorable reaction so that the overall free- energy change for the two reactions together is favorable Coupled reactions Imagine that reaction 1 does not occur to any reasonable extent because it has a small equilibrium constant and is energetically unfavorable,the reaction has AG 0 (1)A+m二B+n△G>0 where A and B are the biochemically "interesting"substances undergoing transformation,while m and n are enzyme cofactors, H2O,or other substances An energetically unfavorable reaction “couples” with an energetically favorable reaction so that the overall freeenergy change for the two reactions together is favorable Coupled reactions ▪ Imagine that reaction 1 does not occur to any reasonable extent because it has a small equilibrium constant and is energetically unfavorable, the reaction has ∆G > 0 (1) A + m B + n ∆G > 0 where A and B are the biochemically “interesting” substances undergoing transformation, while m and n are enzyme cofactors, H2O, or other substances An Overview of Metabolism and Biochemical Energy