正在加载图片...
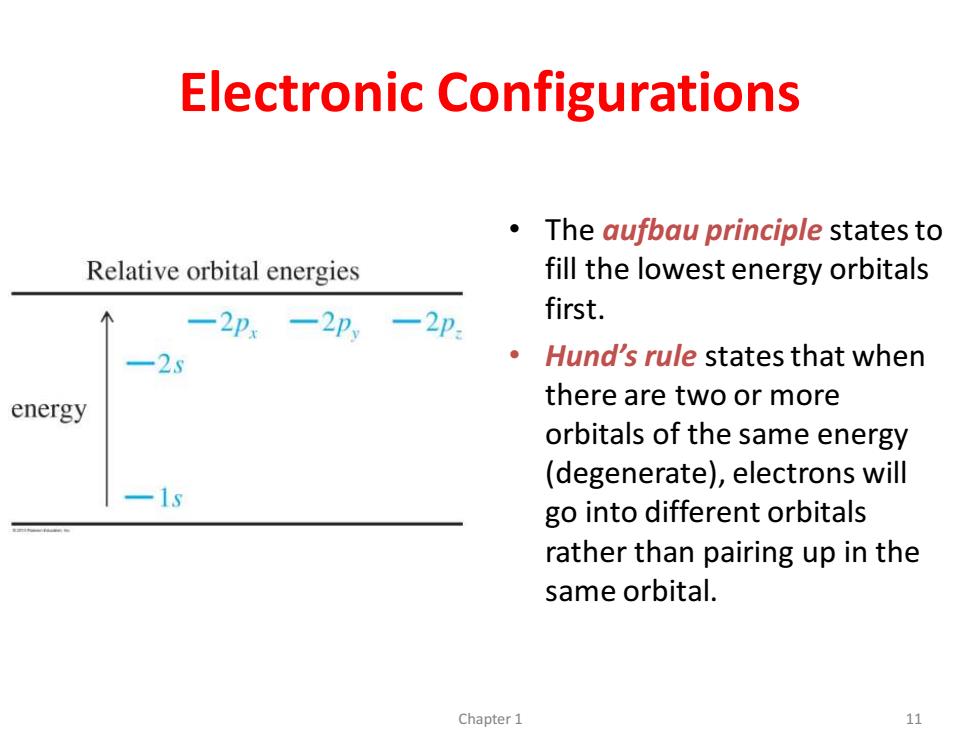
Electronic Configurations The aufbau principle states to Relative orbital energies fill the lowest energy orbitals 一2p,-2p一2p first. -25 Hund's rule states that when energy there are two or more orbitals of the same energy (degenerate),electrons will -1s go into different orbitals rather than pairing up in the same orbital. Chapter 1 11Electronic Configurations • The aufbau principle states to fill the lowest energy orbitals first. • Hund’s rule states that when there are two or more orbitals of the same energy (degenerate), electrons will go into different orbitals rather than pairing up in the same orbital. Chapter 1 11