正在加载图片...
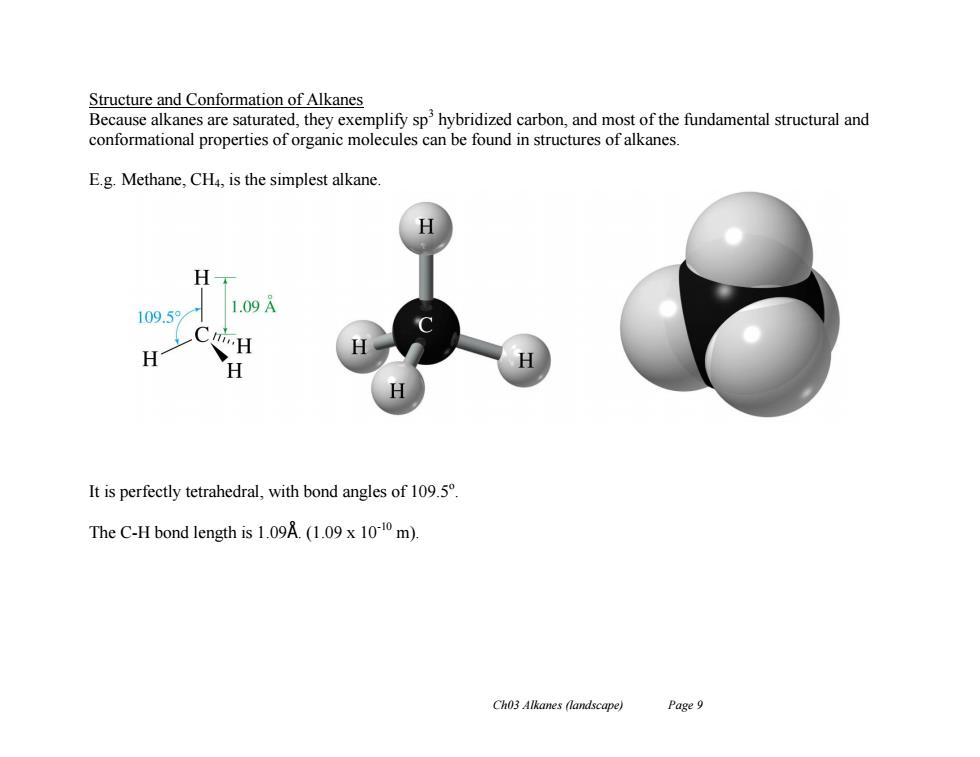
Structure and Conformation of Alkanes Because alkanes are saturated,they exemplify sphybridized carbon,and most of the fundamental structural and conformational properties of organic molecules can be found in structures of alkanes. E.g.Methane,CH4,is the simplest alkane. H 109.5 1.09A H H It is perfectly tetrahedral,with bond angles of 109.5 The C-H bond length is 1.09A.(1.09 x 1010 m). Ch03 Alkanes (landscape) Page 9 Ch03 Alkanes (landscape) Page 9 Structure and Conformation of Alkanes Because alkanes are saturated, they exemplify sp3 hybridized carbon, and most of the fundamental structural and conformational properties of organic molecules can be found in structures of alkanes. E.g. Methane, CH4, is the simplest alkane. It is perfectly tetrahedral, with bond angles of 109.5o . The C-H bond length is 1.09Å. (1.09 x 10-10 m)