正在加载图片...
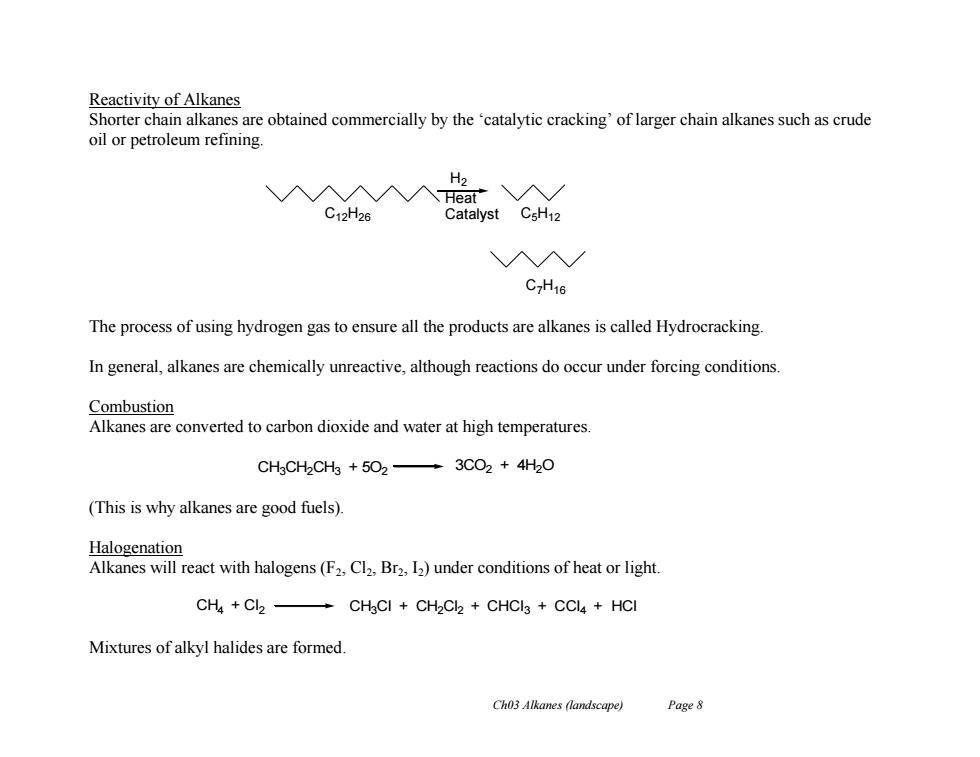
Reactivity of Alkanes Shorter chain alkanes are obtained commercially by the 'catalytic cracking'of larger chain alkanes such as crude oil or petroleum refining H2 入A入入Heat"入/ C12H26 Catalyst CsH12 入N/ C7H16 The process of using hydrogen gas to ensure all the products are alkanes is called Hydrocracking. In general,alkanes are chemically unreactive,although reactions do occur under forcing conditions Combustion Alkanes are converted to carbon dioxide and water at high temperatures. CHgCH2CH3 +502-3CO2 4H2O (This is why alkanes are good fuels). Halogenation Alkanes will react with halogens(F2,Cl2,Br2,12)under conditions of heat or light. CH4 Cl2- CH3Cl CH2Cl2 CHCI3 CCl4 HCI Mixtures of alkyl halides are formed. Ch03 Alkanes (landscape) Page 8Ch03 Alkanes (landscape) Page 8 Reactivity of Alkanes Shorter chain alkanes are obtained commercially by the ‘catalytic cracking’ of larger chain alkanes such as crude oil or petroleum refining. The process of using hydrogen gas to ensure all the products are alkanes is called Hydrocracking. In general, alkanes are chemically unreactive, although reactions do occur under forcing conditions. Combustion Alkanes are converted to carbon dioxide and water at high temperatures. (This is why alkanes are good fuels). Halogenation Alkanes will react with halogens (F2, Cl2, Br2, I2) under conditions of heat or light. Mixtures of alkyl halides are formed. H2 Heat C12H26 Catalyst C5H12 C7H16 CH4 + Cl2 CH3Cl + CH2Cl2 + CHCl3 + CCl4 + HCl CH3CH2CH3 + 5O2 3CO2 + 4H2O