正在加载图片...
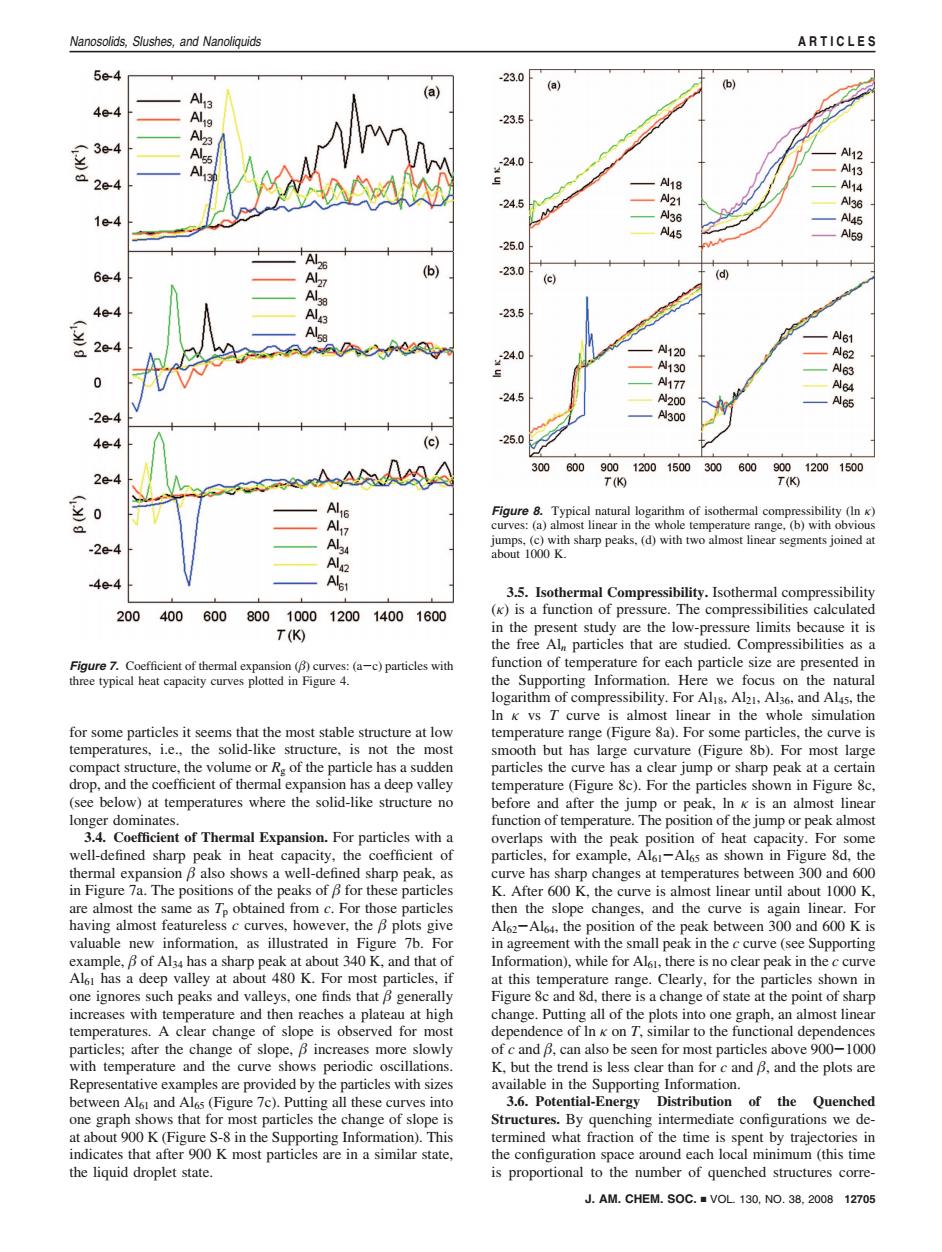
Nanosolids,Slushes,and Nanoliquids ARTICLES 5e4 -23.0 (a) 6 4e4 23.5 e -24.0 2e-4 ,N18 A14 -24.5 A21 Al36 A45 -25.0 (b) 23.0 (c) -23.5 A61 -24.0 AN120 A62 AN130 Al63 AN177 Al64 -24.5 AN200 A65 N300 (c) -25.0 300 600 900 12001500 300 600 9001200 1500 T(9 T(K) Figure 8.Typical natural logarithm of isothermal compressibility (In K) curves:(a)almost linear in the whole temperature range,(b)with obvious jumps,(c)with sharp peaks.(d)with two almost linear segments joined at about 1000 K. Alet 3.5.Isothermal Compressibility.Isothermal compressibility 200400 600 8001000120014001600 (K)is a function of pressure.The compressibilities calculated T(K) in the present study are the low-pressure limits because it is the free Al particles that are studied.Compressibilities as a Figure 7.Coefficient of thermal expansion (B)curves:(a-c)particles with function of temperature for each particle size are presented in three typical heat capacity curves plotted in Figure 4. the Supporting Information.Here we focus on the natural logarithm of compressibility.For Al1s.Al2,Al36.and Al4s,the In k vs T curve is almost linear in the whole simulation for some particles it seems that the most stable structure at low temperature range (Figure 8a).For some particles,the curve is temperatures,i.e.,the solid-like structure,is not the most smooth but has large curvature (Figure 8b).For most large compact structure,the volume or R.of the particle has a sudden particles the curve has a clear jump or sharp peak at a certain drop,and the coefficient of thermal expansion has a deep valley temperature(Figure 8c).For the particles shown in Figure 8c, (see below)at temperatures where the solid-like structure no before and after the jump or peak,In k is an almost linear longer dominates. function of temperature.The position of the jump or peak almost 3.4.Coefficient of Thermal Expansion.For particles with a overlaps with the peak position of heat capacity.For some well-defined sharp peak in heat capacity,the coefficient of particles,for example,Al61-Al6s as shown in Figure 8d,the thermal expansion B also shows a well-defined sharp peak,as curve has sharp changes at temperatures between 300 and 600 in Figure 7a.The positions of the peaks of B for these particles K.After 600 K,the curve is almost linear until about 1000 K, are almost the same as Tp obtained from c.For those particles then the slope changes,and the curve is again linear.For having almost featureless c curves,however,the B plots give Al62-Al64,the position of the peak between 300 and 600 K is valuable new information,as illustrated in Figure 7b.For in agreement with the small peak in the c curve (see Supporting example,B of Al34 has a sharp peak at about 340 K,and that of Information),while for Al61,there is no clear peak in the c curve Al6 has a deep valley at about 480 K.For most particles,if at this temperature range.Clearly,for the particles shown in one ignores such peaks and valleys,one finds that B generally Figure 8c and 8d,there is a change of state at the point of sharp increases with temperature and then reaches a plateau at high change.Putting all of the plots into one graph,an almost linear temperatures.A clear change of slope is observed for most dependence of In k on T,similar to the functional dependences particles;after the change of slope,B increases more slowly of c and B,can also be seen for most particles above 900-1000 with temperature and the curve shows periodic oscillations. K,but the trend is less clear than for c and B,and the plots are Representative examples are provided by the particles with sizes available in the Supporting Information. between Al61 and Al6s(Figure 7c).Putting all these curves into 3.6.Potential-Energy Distribution of the Quenched one graph shows that for most particles the change of slope is Structures.By quenching intermediate configurations we de- at about 900 K(Figure S-8 in the Supporting Information).This termined what fraction of the time is spent by trajectories in indicates that after 900 K most particles are in a similar state, the configuration space around each local minimum (this time the liquid droplet state. is proportional to the number of quenched structures corre- J.AM.CHEM.SOC.VOL 130,NO.38,2008 12705for some particles it seems that the most stable structure at low temperatures, i.e., the solid-like structure, is not the most compact structure, the volume or Rg of the particle has a sudden drop, and the coefficient of thermal expansion has a deep valley (see below) at temperatures where the solid-like structure no longer dominates. 3.4. Coefficient of Thermal Expansion. For particles with a well-defined sharp peak in heat capacity, the coefficient of thermal expansion also shows a well-defined sharp peak, as in Figure 7a. The positions of the peaks of for these particles are almost the same as Tp obtained from c. For those particles having almost featureless c curves, however, the plots give valuable new information, as illustrated in Figure 7b. For example, of Al34 has a sharp peak at about 340 K, and that of Al61 has a deep valley at about 480 K. For most particles, if one ignores such peaks and valleys, one finds that generally increases with temperature and then reaches a plateau at high temperatures. A clear change of slope is observed for most particles; after the change of slope, increases more slowly with temperature and the curve shows periodic oscillations. Representative examples are provided by the particles with sizes between Al61 and Al65 (Figure 7c). Putting all these curves into one graph shows that for most particles the change of slope is at about 900 K (Figure S-8 in the Supporting Information). This indicates that after 900 K most particles are in a similar state, the liquid droplet state. 3.5. Isothermal Compressibility. Isothermal compressibility (κ) is a function of pressure. The compressibilities calculated in the present study are the low-pressure limits because it is the free Aln particles that are studied. Compressibilities as a function of temperature for each particle size are presented in the Supporting Information. Here we focus on the natural logarithm of compressibility. For Al18, Al21, Al36, and Al45, the ln κ vs T curve is almost linear in the whole simulation temperature range (Figure 8a). For some particles, the curve is smooth but has large curvature (Figure 8b). For most large particles the curve has a clear jump or sharp peak at a certain temperature (Figure 8c). For the particles shown in Figure 8c, before and after the jump or peak, ln κ is an almost linear function of temperature. The position of the jump or peak almost overlaps with the peak position of heat capacity. For some particles, for example, Al61-Al65 as shown in Figure 8d, the curve has sharp changes at temperatures between 300 and 600 K. After 600 K, the curve is almost linear until about 1000 K, then the slope changes, and the curve is again linear. For Al62-Al64, the position of the peak between 300 and 600 K is in agreement with the small peak in the c curve (see Supporting Information), while for Al61, there is no clear peak in the c curve at this temperature range. Clearly, for the particles shown in Figure 8c and 8d, there is a change of state at the point of sharp change. Putting all of the plots into one graph, an almost linear dependence of ln κ on T, similar to the functional dependences of c and , can also be seen for most particles above 900-1000 K, but the trend is less clear than for c and , and the plots are available in the Supporting Information. 3.6. Potential-Energy Distribution of the Quenched Structures. By quenching intermediate configurations we determined what fraction of the time is spent by trajectories in the configuration space around each local minimum (this time is proportional to the number of quenched structures correFigure 7. Coefficient of thermal expansion () curves: (a-c) particles with three typical heat capacity curves plotted in Figure 4. Figure 8. Typical natural logarithm of isothermal compressibility (ln κ) curves: (a) almost linear in the whole temperature range, (b) with obvious jumps, (c) with sharp peaks, (d) with two almost linear segments joined at about 1000 K. J. AM. CHEM. SOC. 9 VOL. 130, NO. 38, 2008 12705 Nanosolids, Slushes, and Nanoliquids ARTICLES