正在加载图片...
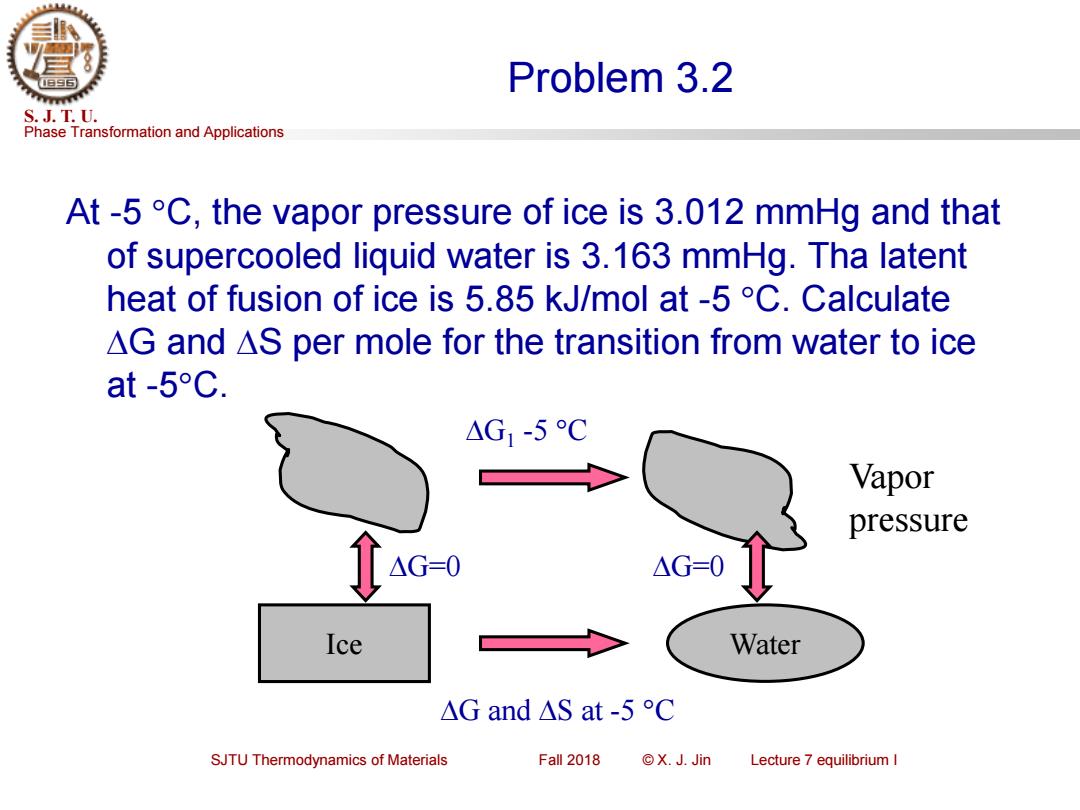
Problem 3.2 S.J.T.U. Phase Transformation and Applications At-5 C,the vapor pressure of ice is 3.012 mmHg and that of supercooled liquid water is 3.163 mmHg.Tha latent heat of fusion of ice is 5.85 kJ/mol at -5C.Calculate AG and AS per mole for the transition from water to ice at-5°C. △G1-5C Vapor pressure △G=0 △G=-0 Ice Water △Gand△Sat-5C SJTU Thermodynamics of Materials Fall 2018 ©X.J.Jin Lecture 7 equilibrium IPhase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Fall 2018 © X. J. Jin Lecture 7 equilibrium I Problem 3.2 At -5 C, the vapor pressure of ice is 3.012 mmHg and that of supercooled liquid water is 3.163 mmHg. Tha latent heat of fusion of ice is 5.85 kJ/mol at -5 C. Calculate G and S per mole for the transition from water to ice at -5C. Ice Water G and S at -5 C G1 -5 C G=0 G=0 Vapor pressure