正在加载图片...
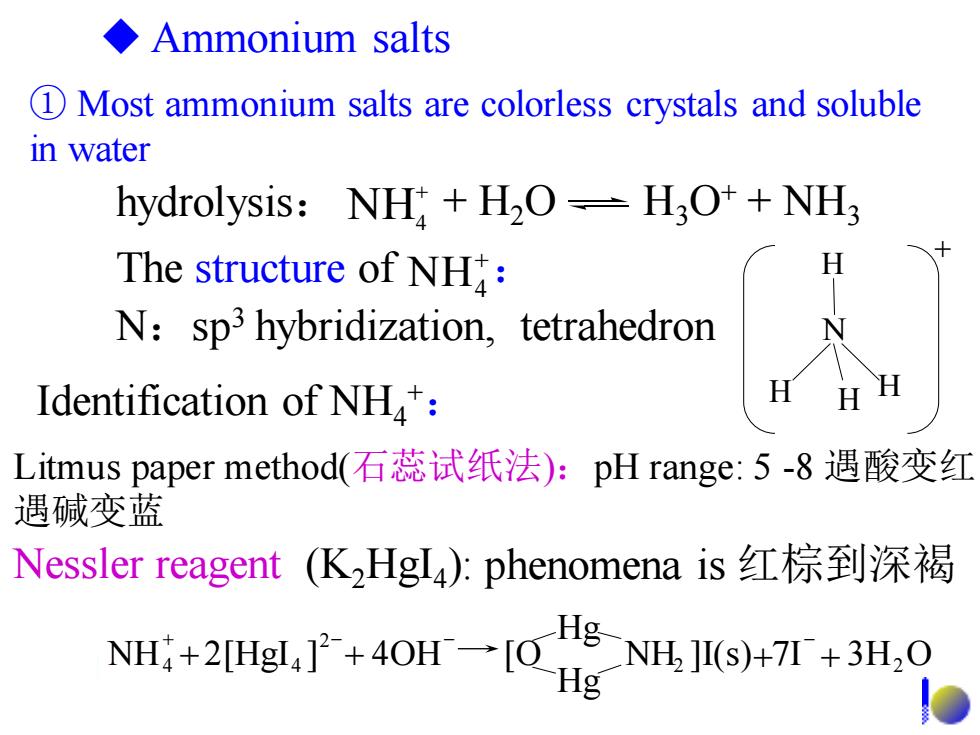
◆Ammonium salts 1 Most ammonium salts are colorless crystals and soluble in water hydrolysis:NH H2O=H3O++NH3 The structure of NH: N:sp3 hybridization,tetrahedron Identification of NH: H Litmus paper method(石蕊试纸法):pH range:5-8遇酸变红 遇碱变蓝 Nessler reagent(K2HgI4:phenomena is红棕到深褐 NH+2Hg,广+40H一OHg g-NH.HI(s)+I+3H.O ① Most ammonium salts are colorless crystals and soluble in water H N H H H + ◆ Ammonium salts Litmus paper method(石蕊试纸法):pH range: 5 -8 遇酸变红, 遇碱变蓝 + NH4 The structure of : Nessler reagent (K2HgI4 ): phenomena is 红棕到深褐 N:sp3 hybridization, tetrahedron Identification of NH4 +: NH ]I(s) 7I 3H O Hg Hg NH 2[HgI ] 4OH [O 2 2 2 4 + 4 + + + + - - - hydrolysis: + H2O H3O+ + NH3 + NH4